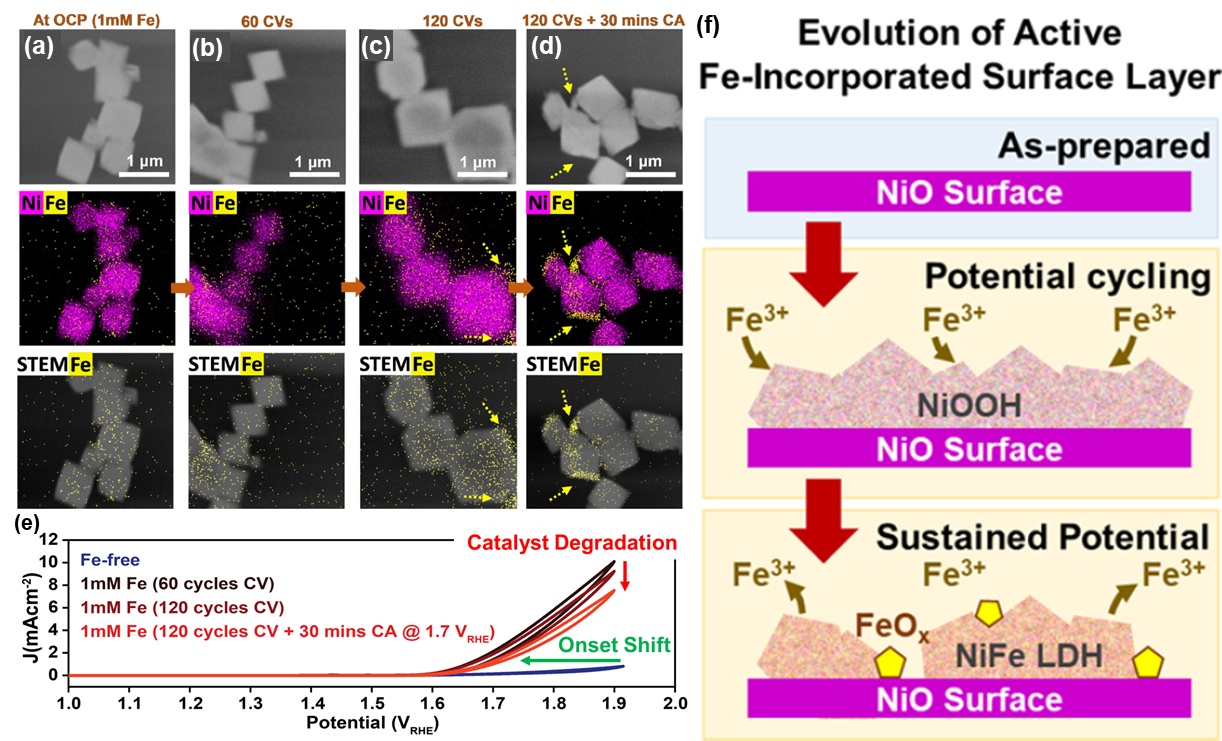
Fe incorporation into NiO particles resolved using in-situ TEM
Researchers at the Fritz-Haber-Institut der Max-Planck-Gesellschaft published recent work demonstrating the powerful capability of the Hummingbird Scientific Gen V In-situ Bulk Liquid-electrochemistry TEM sample holder to characterize the evolution of octahedral NiO catalyst particles under repeated high-cycle electrochemical cycling in a liquid cell infused with Fe-containing electrolyte. The electrochemical response for the holder re-produces the expected shift in OER onset potential due to Fe impurities in the electrolyte changing the chemistry of the catalyst. These results clarify the structural evolution of a NiO surface during OER and the critical role of Ni-Fe layered double hydroxide formation and degradation in determining the electrocatalytic performance.
Figure: (a-d) In-situ STEM images, combined Ni/Fe chemical EDS maps and STEM/Fe EDS maps of NiO octahedra during and after CV cycling. (e) Cyclic voltammograms collected before and after Fe addition and CV cycling. (f) Schematic of two-stage mechanism for Fe incorporation into NiO octahedra.
Image Copyright © 2023 The Authors. Published by American Chemical Society.
Reference: Yang et. al. J. Am. Chem. Soc. 145 (39) 21465-21474 (2023) DOI: 10.1021/jacs.3c07158