Synthesis
| Gas-Heating TEM | Liquid-Heating-Optical TEM | Heating-Biasing TEM | Tomography TEM | Liquid X-Ray | ||
| Stimuli |
Electrical |  |
 |
 |
 |
 |
| Thermal |  |
 |
 |
 |
 |
|
| Optical |  |
 |
 |
 |
 |
|
| Imaging | Higher resolution and diffraction |  |
 |
 |
 |
 |
| EDS/EELS compatibility |  |
 |
 |
 |
 |
|
| 3D reconstruction |  |
 |
 |
 |
 |
|
| In-situ imaging |  |
 |
 |
 |
 |
|
| Pre-and post-mortem analysis |  |
 |
 |
 |
 |
|
| Environment |
Liquid |  |
 |
 |
 |
 |
| Gas |  |
 |
 |
 |
 |
|
| Vacuum |  |
 |
 |
 |
 |
|
 Excellent
Excellent  Good
Good  N/A
N/A
The Origins of a Mineral
In a study published in Science, researchers at Lawrence Berkeley, Pacific Northwest National Laboratories, and the University of Washington used Hummingbird Scientific’s dual-flow liquid-TEM sample holder to directly observe calcium carbonate crystal nucleation. Their research reveals the presence of multiple simultaneously-operating nucleation pathways and calls into question traditional assumptions about the nature of the nucleation process. “For a decade, we’ve been studying the formation pathways of carbonates using high-powered microscopes,” explains Dr. Jim DeYoreo, the project lead, in a PNNL press release. “But we hadn’t had the tools to watch the crystals form in real time. Now we know the pathways are far more complicated than envisioned in the models established in the twentieth century.”
Because calcium carbonate is the largest global carbon sink, the results of this study have particular relevance to climatologists, who could use them to help explain the processes through which carbon dioxide is sequestered in rocks, minerals, shells, and reefs. In future work, Dr. DeYoreo and his fellow researchers hope to observe living organisms’ roles in calcium carbonate nucleation.
Reference: M.H. Nielsen, S. Aloni, and J.J. De Yoreo. “In-situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways,” Science 345:6201 (2014) pp. 1158–1162 Abstract
Copyright © 2014, American Association for the Advancement of Science
Edit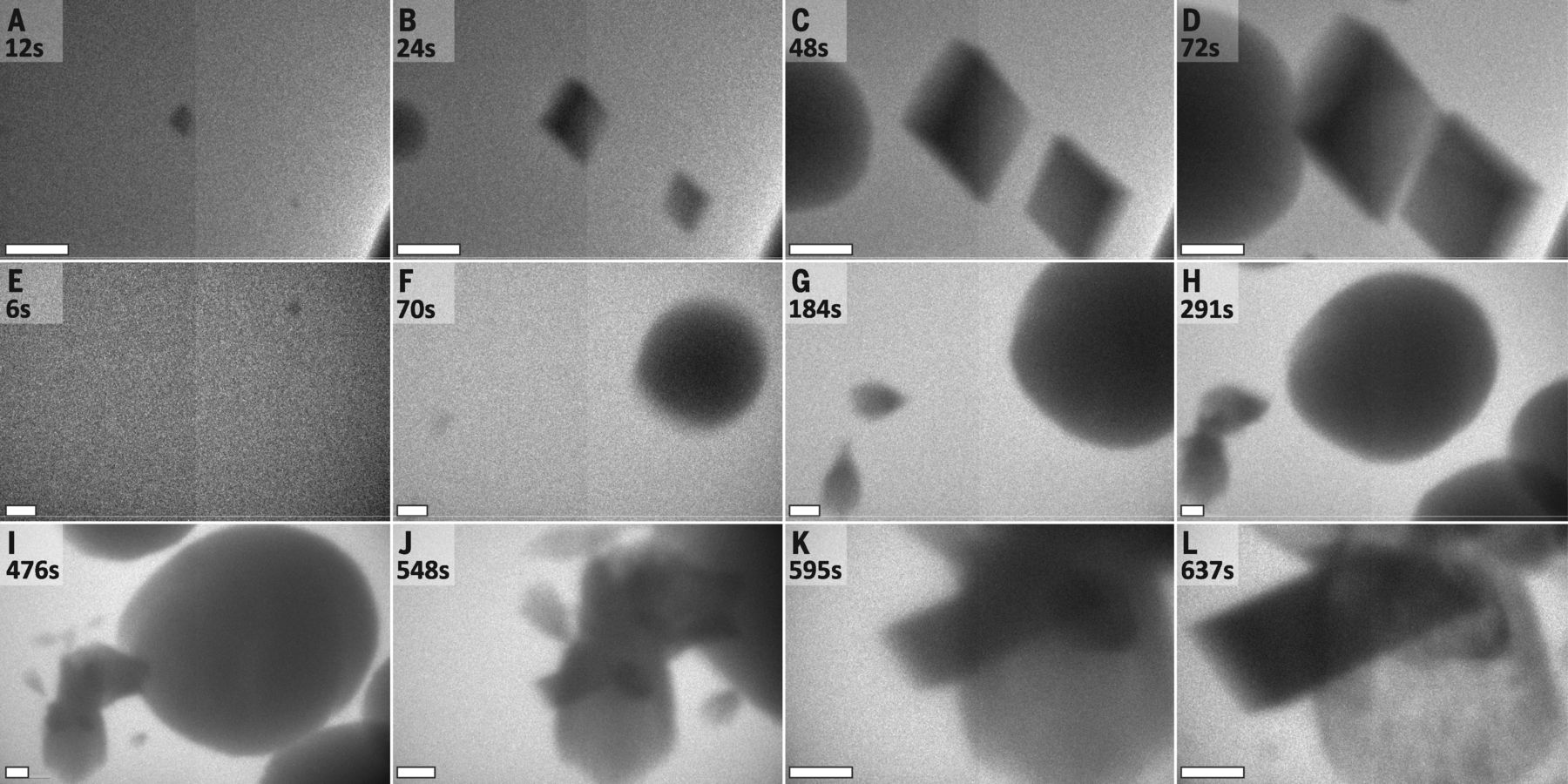
Concurrent formation of multiple phases. All scale bars are 500nm. Image courtesy of M.H. Nielson et al. Copyright © 2014, American Association for the Advancement of Science.
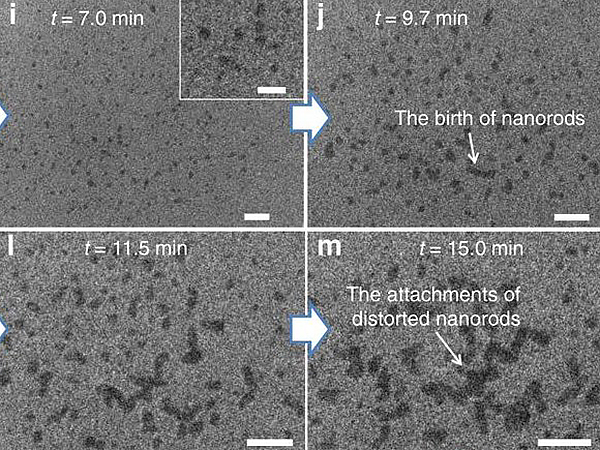
Self-Assembly of Branched Nanocrystals
Inverted dark-field STEM images showing the formation of ordered chains of CdSe/CdS octapods in toluene. The video shows the evolution of the system starting from single octapods dispersed in the entire liquid volume to interlocked chain-like structures.
Left: Total width and height of video frame is 3.64 μm x 3.64 μm
References: Eli Sutter, Peter Sutter, Alexei V. Tkachenko, Roman Krahne, Joost de Graaf, Milena Arciniegas and Liberato Manna. “In situ microscopy of the self-assembly of branched nanocrystals in solution,” Nature Communications (2016) Abstract
Video Copyright © 2018 Springer Nature Limited
Top page banner: Video Copyright © 2018 Springer Nature Limited
Edit| Shu Fen Tan, Geeta Bisht, Utkarsh Anand, Michel Bosman, Xin Ee Yong, and Utkur Mirsaidov. “In situ Kinetic and Thermodynamic Growth Control of Au-Pd Core-Shell Nanoparticles.” Journal of the American Chemical Society (2018) | Abstract |
| Jeffery A. Aguiar, Nooraldeen R. Alkurd, Sarah Wozny, Maulik K. Patel, Mengjin Yang, Weilie Zhou, Mowafak Al-Jassim, Terry G. Holesinger, Kai Zhu and Joseph J. Berry. “In situ investigation of halide incorporation into perovskite solar cells,” MRS Communications (2017) | Abstract |
| Jeffery A. Aguiar, Sarah Wozny, Terry G. Holesinger, Toshihiro Aoki,d Maulik K. Patel, Mengjin Yang, Joseph J. Berry, Mowafak Al-Jassim, Weilie Zhou and Kai Zhu. “In situ investigation of the formation and metastability of formamidinium lead tri-iodide perovskite solar cells,” Energy & Environmental Science (2016) | Abstract |
| Dongdong Xiao, Zhigang Wu, Miao Song, Jaehun Chun, Gregory K. Schenter, and Dongsheng Li. “Silver Nanocube and Nanobar Growth via Anisotropic Monomer Addition and Particle Attachment Processes,” Langmuir (2017) | Abstract |
| Lili Liu, Shuai zhang, Mark E. Bowden, Jharna Chaudhuri, and James J. De Yoreo. “In-situ TEM and AFM investigation of morphological controls during the growth of single crystal BaWO4,” Crystal Growth & Design (2017) | Abstract |
| YuBo Wang, Shuai Wang, and Xing Lu. “In Situ Observation of the Growth of ZnO Nanostructures Using Liquid Cell Electron Microscopy,” The Journal of Physical Chemistry C (2017) | Abstract |
| Mingyuan Ge, Ming Lu, Yong Chu & Huolin Xin. “Anomalous Growth Rate of Ag Nanocrystals Revealed by in situ STEM,” Scientific Reports (2017) | Abstract |
| Lucas R. Parent, Evangelos Bakalis, Abelardo Ramírez-Hernández, Jacquelin K. Kammeyer, Chiwoo Park, Juan de Pablo, Francesco Zerbetto, Joseph P. Patterson, and Nathan C. Gianneschi.”Directly Observing Micelle Fusion and Growth in Solution by Liquid-Cell Transmission Electron Microscopy,” Journal of the American Chemical Society (2017) | Abstract |
| E. Sutter, P. Sutter, A. V. Tkachenko, R. Krahne, J. De Graff, M. Arciniegas and L. Manna. “In situ microscopy of the self-assembly of branched nanocrystals in solution,” Nature Communications (2016) | Abstract |
| J.H. Park, N.M. Schneider, J.M. Grogan, M.C. Reuter, H.H. Bau, S. Kodambaka & F.M. Ross. “Control of Electron Beam-Induced Au Nanocrystal Growth Kinetics through Solution Chemistry,” Nano Letters (2015) | Abstract |
| P. J. M. Smeets, K. R. Cho, R. G. E. Kempen, N. A. J. M. Sommerdijk, and J. J. De Yoreo. “Calcium carbonate nucleation driven by ion binding in a biomimetic matrix revealed by in situ electron microscopy”, Nature Materials Letters, Published Online 01/26/2015. | Abstract |
| M.H. Nielsen, S. Aloni, J.J. De Yoreo. “In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways”, Science vol. 345 iss. 6201 (2014) pp. 1158-1162 | Abstract |
| S. Kashyap, T.J. Woehl, X. Liu, S.K. Mallapragada, T. Prozorov. ”Nucleation of Iron Oxide Nanoparticles Mediated by Mms6 Protein In Situ“ ACS Nano (2014) In Print. | Abstract |
| L.R. Parent, D.B. Robinson, P.J. Cappillino, R.J. Hartnett, P. Abellan, J.E. Evans, N.D Browning, and I. Arslan. “In-Situ Observation of Directed Nanoparticle Aggregation During the Synthesis of Ordered Nanoporous Metal in Soft Templates,” Chemistry of Materials 26:3 (2014) pp. 1426‒1433 | Abstract |
| T.J. Woehl, C. Park, J.E. Evans, I. Arslan, W.D. Ristenpart, N.D. Browning. “Direct Observation of Aggregative Nanoparticle Growth: Kinetic Modeling of the Size Distribution and Growth Rate,” Nano Lett. 14 (2014) pp. 373‒378 | Abstract |
| K.L. Jungjohann, S. Bliznakov, P.W. Sutter, E.A. Stach, E.A. Sutter. “In-Situ Liquid Cell Electron Microscopy of the Solution Growth of Au-Pd Core-Shell Nanostructures,” Nano. Lett. 13 (2013) pp. 2964–2970 | Abstract |
| M.H. Nielsen, J.R.I. Lee, Q. Hu, T. Y.-J. Han, and J.J. De Yoreo, “Structural evolution, formation pathways and energetic controls during template-directed nucleation of CaCO3,” Faraday Discuss. 159 (2012) pp. 105–121 | Abstract |
Read More