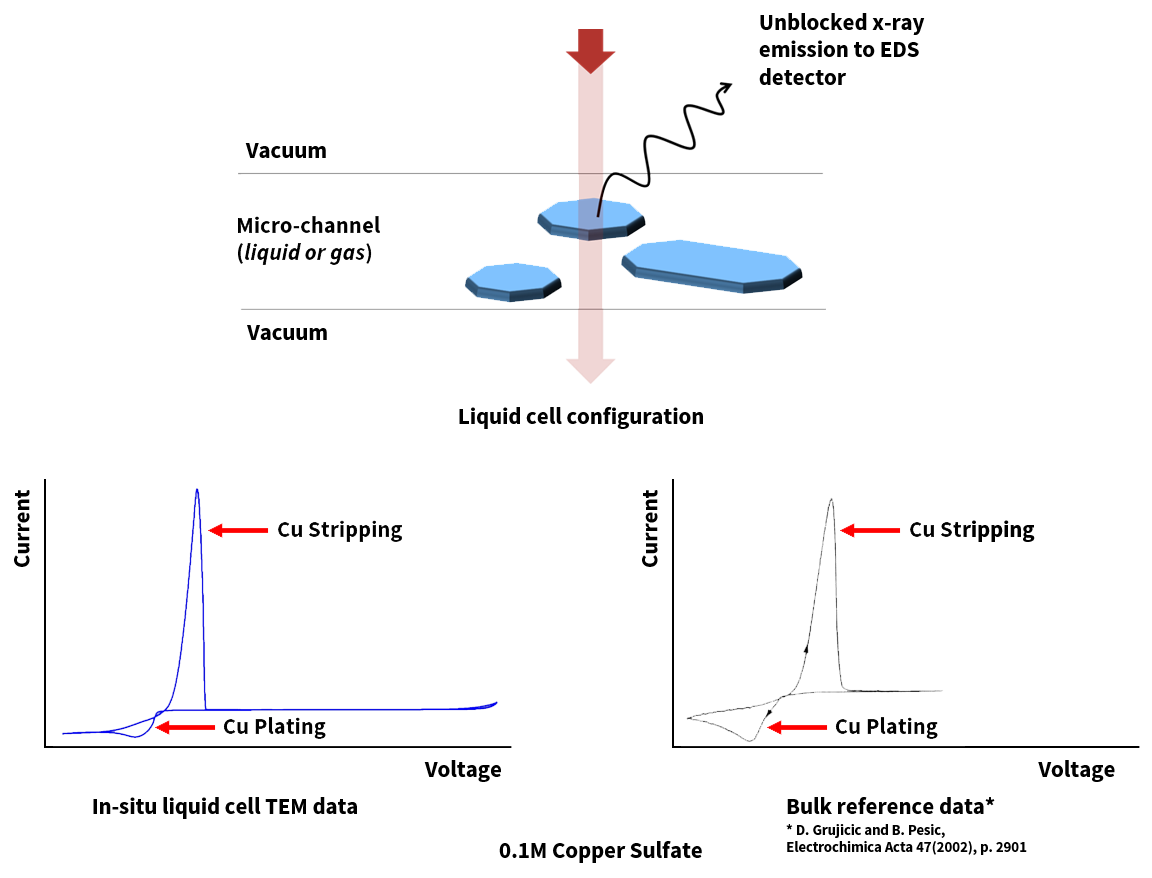
To validate the performance of the Generation V Bulk Electrochemistry holder, we also perform TEM copper electrodeposition and compare the behavior with the beaker level bulk data.
A cyclic voltammetry (CV) study of a model compound 0.1 M CuSO4 showing plating and stripping of copper has been presented here. The copper plating and stripping occur at the Pt working electrode at distinct redox peaks and the result replicate the bulk electrochemical cells with large electrode areas and larger volume of electrolyte solution.
Image Left: Comparison of CV curves between in-situ TEM and bulk reference data.