Operando STXM demonstration of critical surface catalytic activity for hydrogen generation
William Chueh et al. from Stanford University, in collaboration with Hummingbird Scientific, Advanced Light Source (Lawrence Berkeley National Laboratory) and the University of Warwick used the operando STXM cell to demonstrate for the first time the surface site activity of transition metal (oxy) hydroxides with an electrochemical stimulus for developing a promising electrocatalyst for the oxygen evolution reaction (OER). This reaction is key in water splitting to generate hydrogen that can be used to store energy.
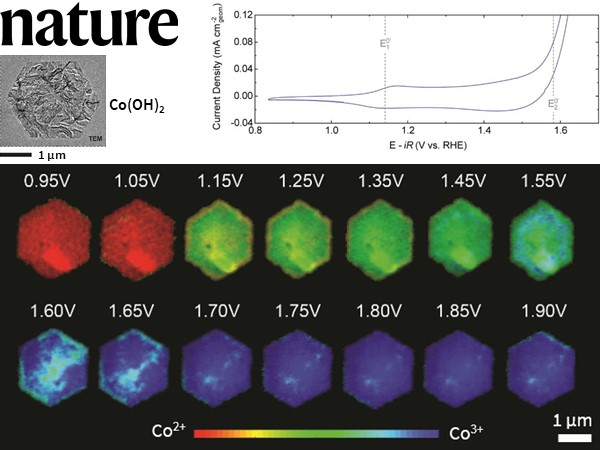
The data from the STXM electrochemical shows heterogeneity in the distribution of Co3+ species in the β-Co(OH)2 particles with an increase in the voltage. However, the electrochemical current is primarily restricted to the particle edge facets. The local concentration of higher Co oxidation state (Co3+) compared to the bulk of the particles suggests that layered oxides’ OER activity can be improved by improvising the surface morphology.
Figure: Cyclic voltammogram in 0.1M KOH of β-Co(OH)2 in the STXM electrochemical flow cell at a scan rate of 10 mV s-1 and a flow rate of 30 μL min-1. The voltage-dependent Co oxidation state phasemap of β-Co(OH)2 is also shown.
Image Copyright © 2021 Springer Nature Limited
Reference: Mefford et al., Nature, 2020 Full Paper