The Origins of a Mineral
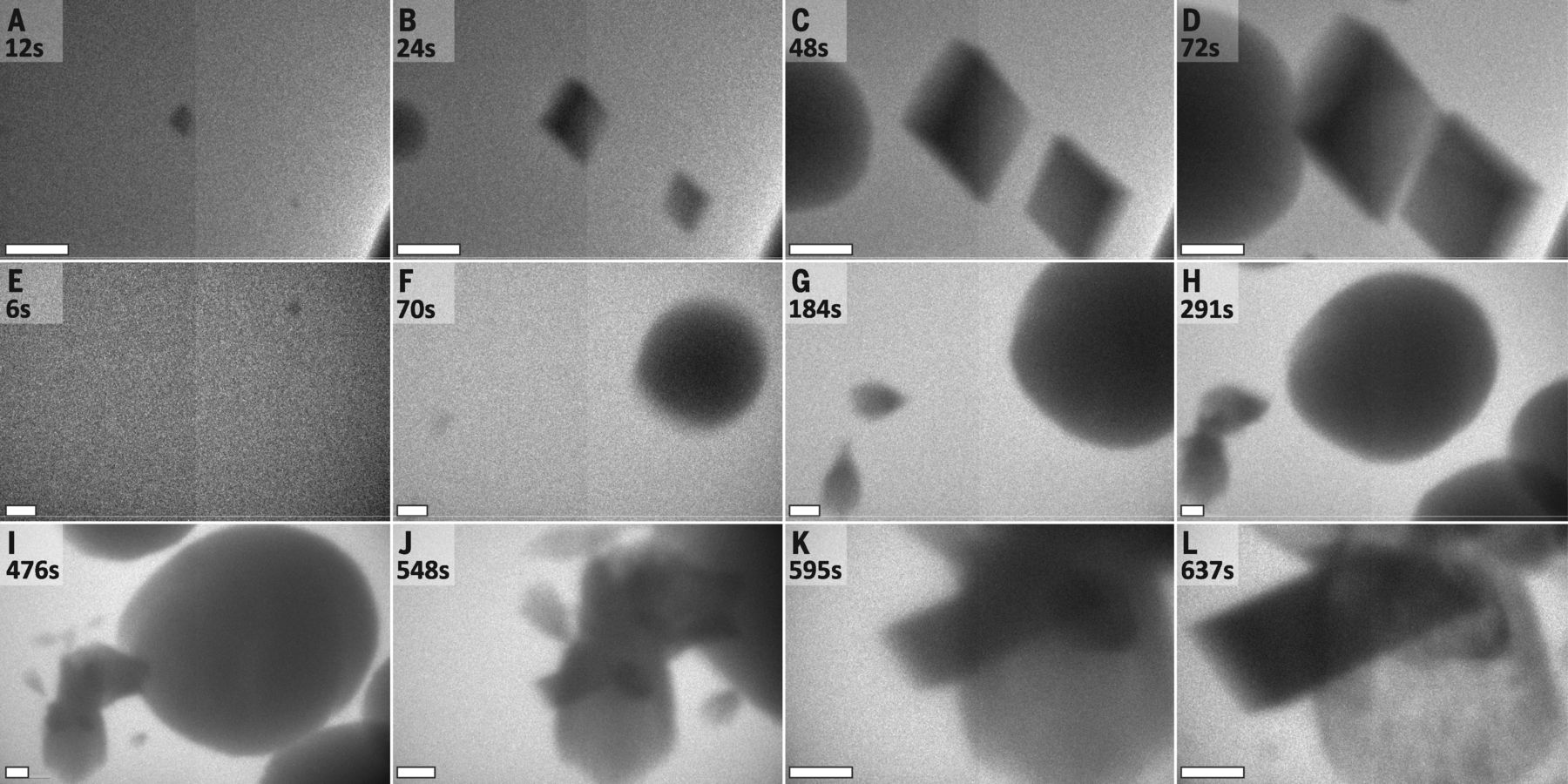
In a study published in Science, researchers at Lawrence Berkeley, Pacific Northwest National Laboratories, and the University of Washington used Hummingbird Scientific’s dual-flow liquid-TEM sample holder to directly observe calcium carbonate crystal nucleation. Their research reveals the presence of multiple simultaneously-operating nucleation pathways and calls into question traditional assumptions about the nature of the nucleation process. “For a decade, we’ve been studying the formation pathways of carbonates using high-powered microscopes,” explains Dr. Jim DeYoreo, the project lead, in a PNNL press release. “But we hadn’t had the tools to watch the crystals form in real time. Now we know the pathways are far more complicated than envisioned in the models established in the twentieth century.”
Because calcium carbonate is the largest global carbon sink, the results of this study have particular relevance to climatologists, who could use them to help explain the processes through which carbon dioxide is sequestered in rocks, minerals, shells, and reefs. In future work, Dr. DeYoreo and his fellow researchers hope to observe living organisms’ roles in calcium carbonate nucleation.
Reference: M.H. Nielsen, S. Aloni, and J.J. De Yoreo. “In-situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways,” Science 345:6201 (2014) pp. 1158–1162 Abstract
Copyright © 2014, American Association for the Advancement of Science