
In-Situ SEM Bulk Liquid Electrochemistry
| 1470 Series SEM | |
| Total Electrodes | 6 |
| True Reference Electrode | Yes* – drift rate less than 0.1 mV/min |
| True Counter Electrode | Yes* |
| Electrolytes | Aqueous, Wide range of organics |
| Spacer Range | 100 nm to 50 μm* |
| Heating Compatibility | Yes |
| SEM Compatibility | Custom integration |
Operando liquid electrochemistry using graphene membrane working electrode
Researchers at EPFL published recent work characterizing graphene as a working electrode for CO2 electroreduction (CO2ER) of Cu nanocatalysts with the Hummingbird Scientific Generation V bulk liquid-electrochemical SEM system. The robust three-layer graphene membrane, which served as the sample carrier, working electrode, and liquid sealing membrane, was applied to the chip via a wet transfer process. Cyclic voltammetry (CV) plots indicated a wider inert cathodic range for the graphene working electrode than glassy carbon. Keeping the probe current low enabled imaging of nanocube degradation and secondary particle deposition during CO2ER at operating potential of -1.1 V vs RHE.
Figure: a) Schematic of graphene membrane preparation via wet transfer process. b) Cyclic voltammetry (CV) and chronoamperometry (CA) of SiNx, graphene, and glassy carbon comparing performance and inert potential range. c-d) Operando SEM, voltammetry and amperometry for CO2ER of Cu nanocatalysts.
Image Copyright © 2024 The Authors. Advanced Materials published by Wiley-VCH GmbH
Reference: Toleukhanova et. al. Adv. Mater. 36 (16) 2311133 (2024) DOI: 10.1021/acs.jpcc.0c09105
Edit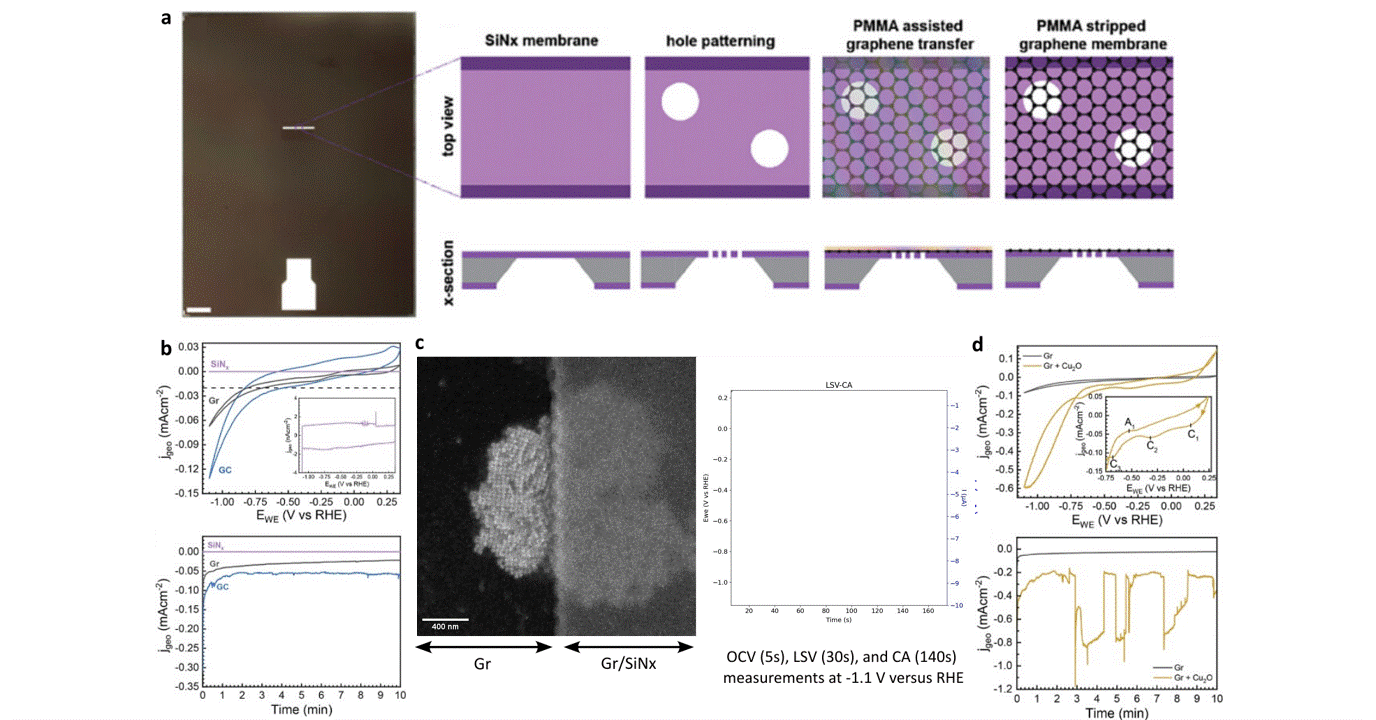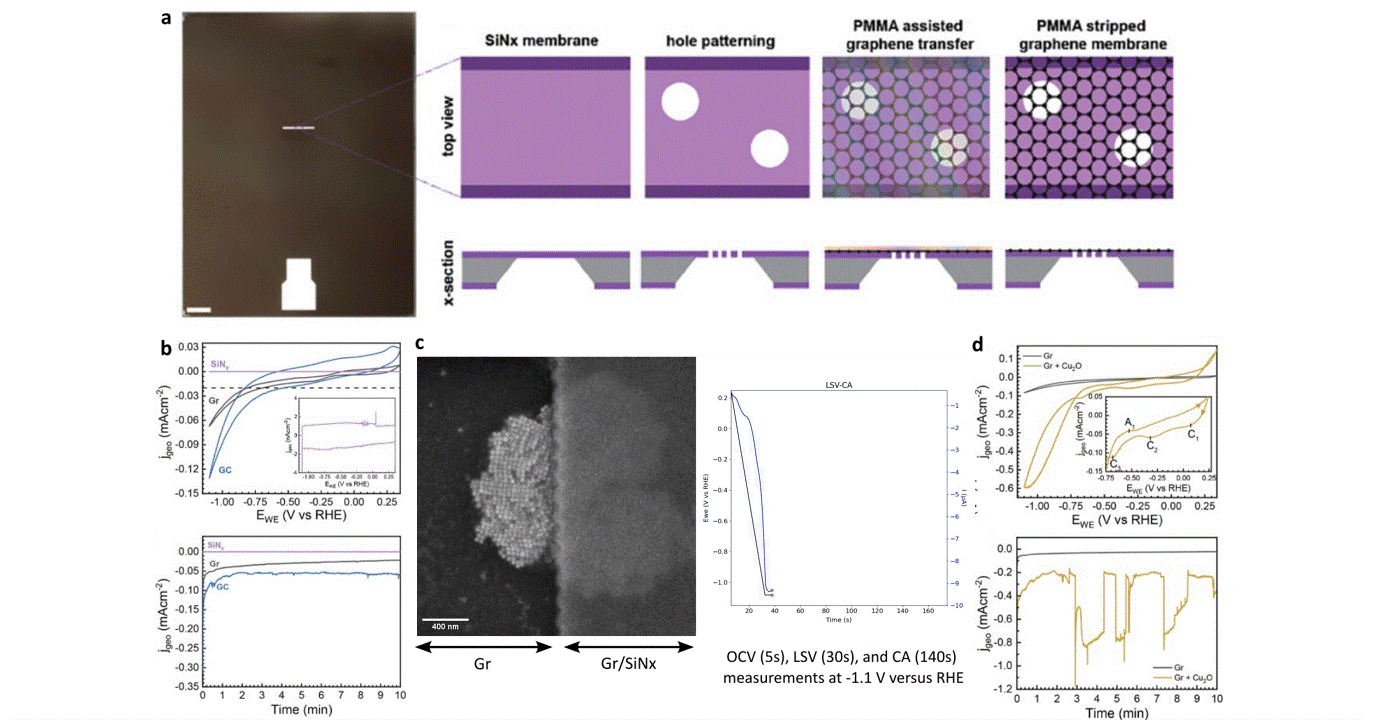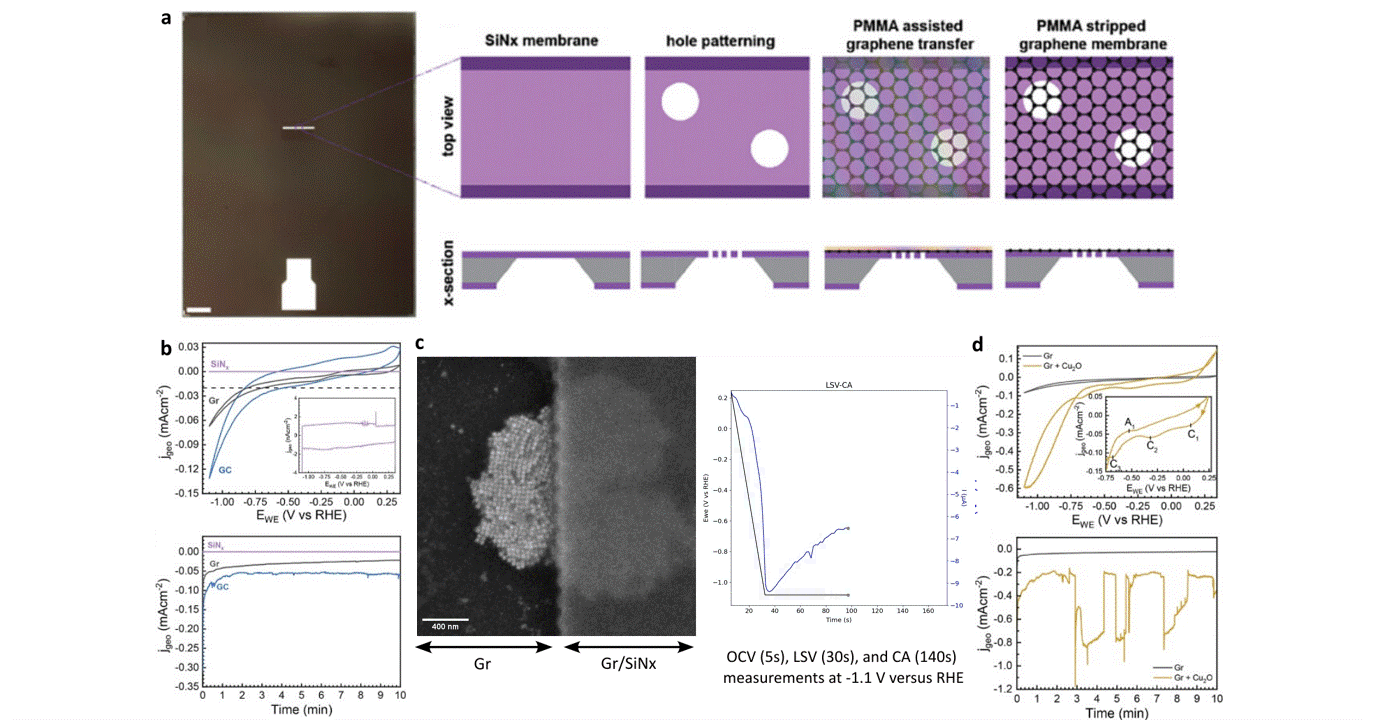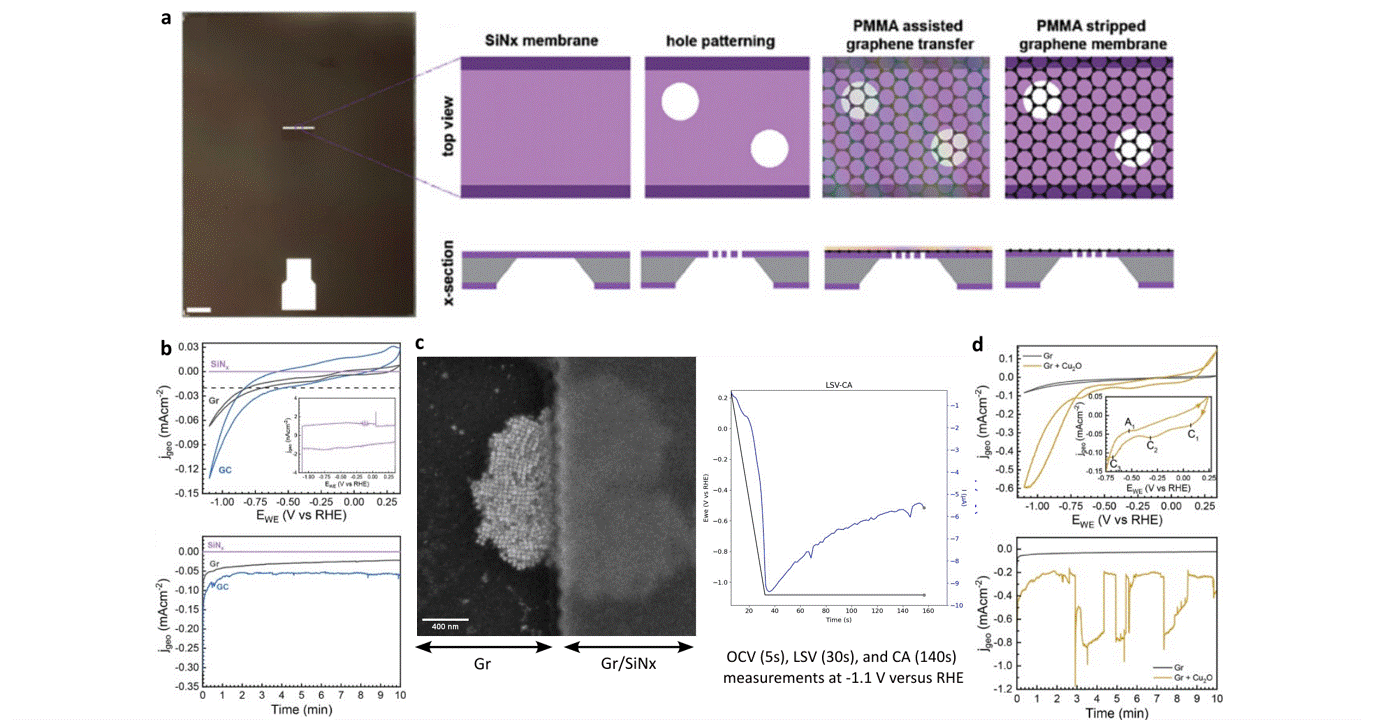
In-situ SEM showing nucleation and growth of Cu cubes during electrochemical cycling
The video on the left published by researchers at Fritz-Haber Institute of the Max Planck Society shows the control nucleation and growth of Cu cubes during a potential sweep. The shape and size of the cubes’ growth correspond with the number of cycles. The result demonstrates that the growth of particles such as Cu cubes can be accurately tuned to achieve optimal sizes and shapes as required for a particular application such as catalysis.
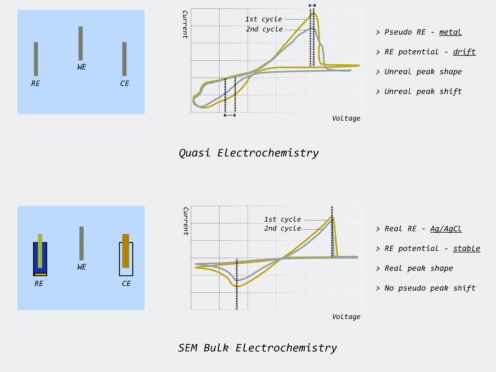
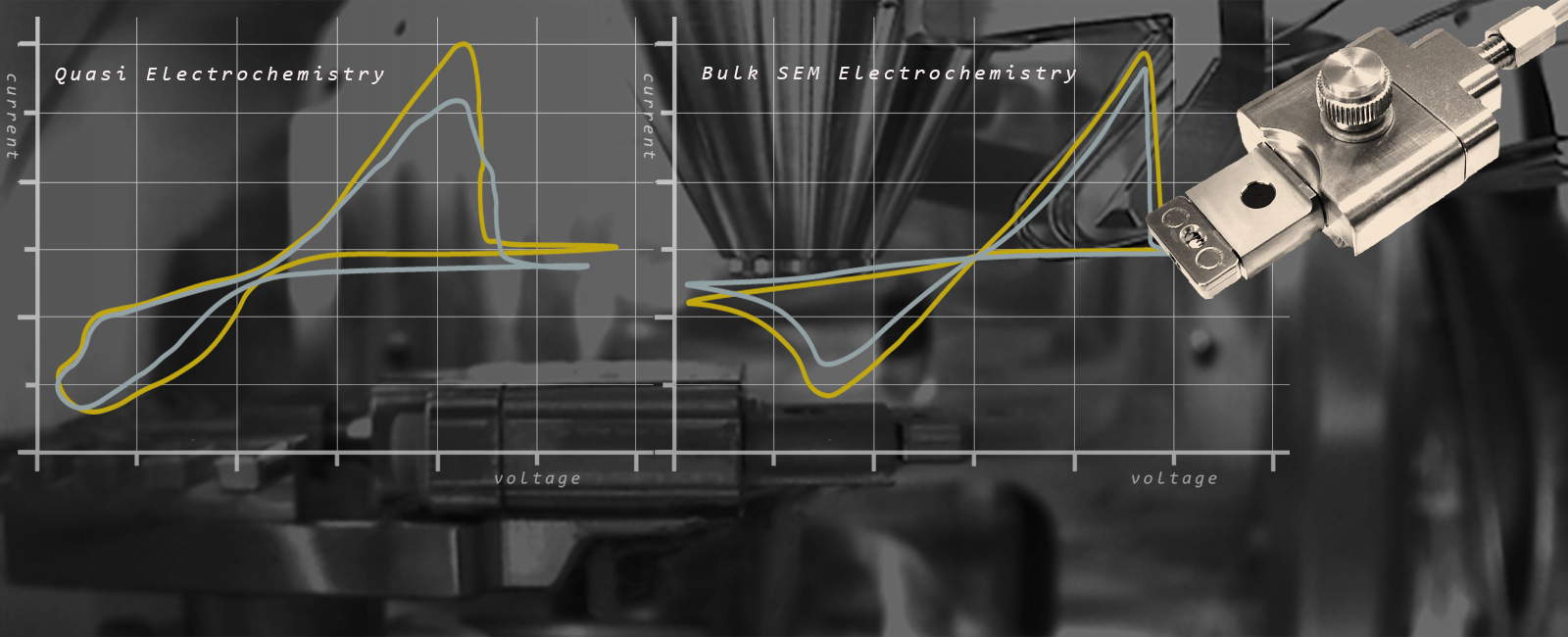
The accuracy in the electrosynthesis of nanoparticles directly correlates to the reliability and accuracy of reference electrodes used over multiple (repeatable) cycles and cannot be achieved with a quasi electrochemistry platform with unrealistic/pseudo reference electrodes.
Video Copyright © 2021 American Chemical Society
Reference: Grosse et al. J. Phys. Chem. C 2020, 124, 49, 26908–26915. DOI: 10.1021/acs.jpcc.0c09105
Edit| Saltanat Toleukhanova, Tzu-Hsien Shen, Chen Chang, Swathilakshmi Swathilakshmi, Tecla Bottinelli Montandon, and Vasiliki Tileli. “Graphene Electrode for Studying CO2 Electroreduction Nanocatalysts under Realistic Conditions in Microcells.” Adv. Mater. (2024) | Abstract |
| Aram Yoon, Antonia Herzog, Philipp Grosse, Daan Hein Alsem, See Wee Chee, and Beatriz Roldán Cuenya. “Dynamic Imaging of Nanostructures in an Electrolyte with a Scanning Electron Microscope.” Microscopy & Microanalysis (2021) | Abstract |
| Philipp Grosse, Aram Yoon, Clara Rettenmaier, See Wee Chee, and Beatriz Roldan Cuenya. “Growth Dynamics and Processes Governing the Stability of Electrodeposited Size-Controlled Cubic Cu Catalysts.” J. Phys. Chem. C (2020) | Abstract |
| See Wee Chee, Aram Yoon, Rosa Aran-Ais, Ruben Rizo, Philipp Grosse, and Beatriz Roldan Cuenya. “Investigating the Behavior of Cu-based Catalysts During Electrochemical CO2 Reduction with Liquid Cell Electron Microscopy.” Microscopy & Microanalysis (2020) | Abstract |
Read More