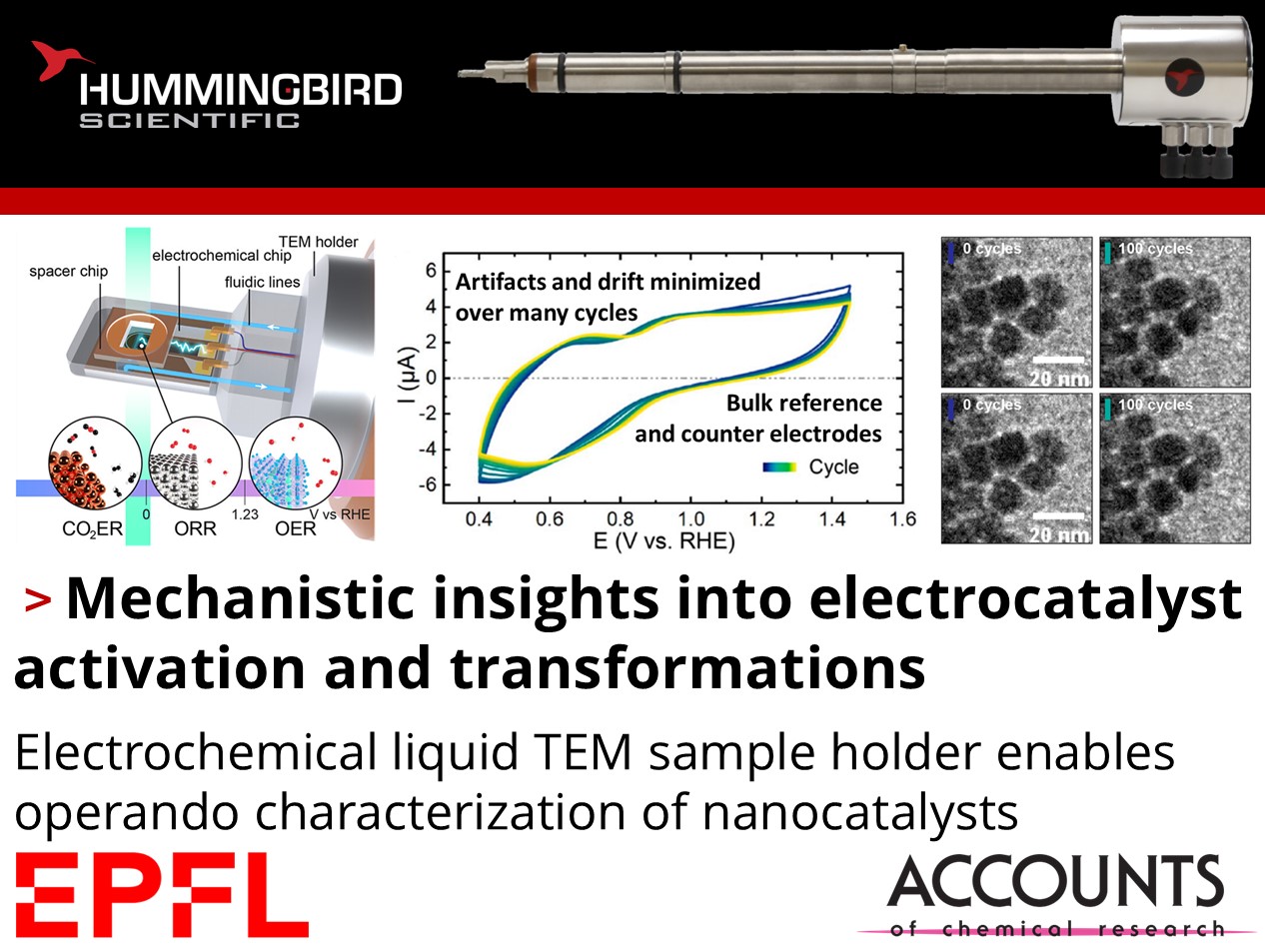
How do nanocatalysts transform during electrochemical reactions?
Tzu-Hsien Shen, Robin Girod, and Vasiliki Tileli from EPFL published a comprehensive account detailing ongoing electrochemical investigations of nanocatalyst transformations using their Hummingbird Scientific Gen V in-situ bulk liquid-electrochemistry TEM sample holder. The team characterized structural transformations of state-of-the-art nanocatalysts under oxygen evolution reaction (OER), oxygen reduction reaction (ORR), and CO2 electroreduction (CO2ER) under in-situ electrochemical liquid-phase transmission electron microscopy (ec-LPTEM), and summarize the latest capabilities, best practices, and associated challenges of such methods.
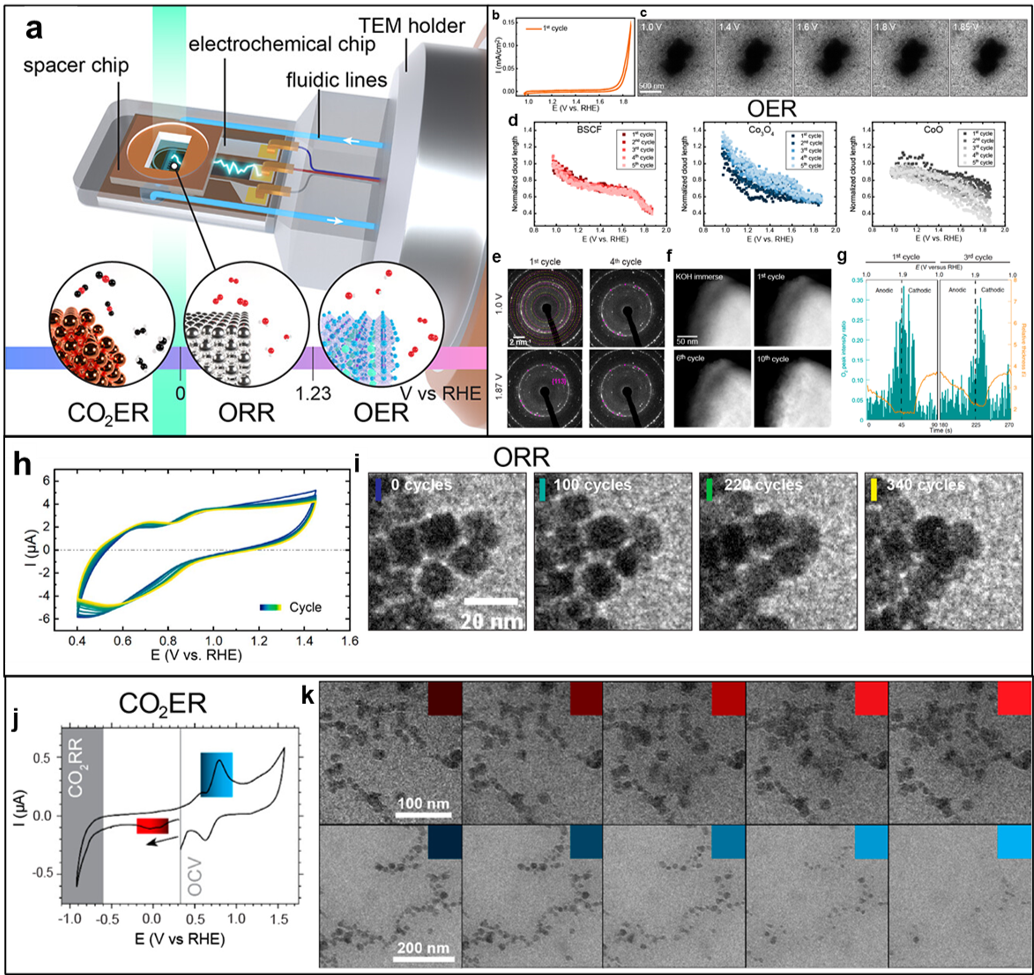
Figures showing a) mockup graphic of Hummingbird Scientific liquid electrochemistry TEM sample holder tip and reactions covered by the summary. b) CV IV plot of first cycle and c) TEM images of BSCF particle at different potential stages for the first cycle of OER. d) Normalized cloud length as a function of applied potential for three Co oxides during OER. e) Selected aperture electron diffraction (SAED) patterns for the first and fourth OER cycles at 1.0 and 1.87 V vs RHE. f) Imaging of the same location of a BSCF particle at various OER cycles. g) O2 peak intensity ratio (green) and EELS-calculated relative thickness (orange curve) with respect to elapsed time (bottom axis) and applied potential (top axis). Copyright 2023 the authors, some rights reserved; exclusive licensee Springer Nature. Distributed under a Creative Commons Attribution License 4.0 (CC BY). h) CV IV plots of 8 nm Pt nanocubes under ORR and i) representative sequences of TEM images with increasing cycles. j) CV IV plots of LSV/CA structures under CO2ER and k) evolution of the secondary particles during cathodic (red) and anodic (blue) potential. Copyright 2023 the authors, some rights reserved; exclusive licensee IOP Publishing. Distributed under a Creative Commons Attribution License 4.0 (CC BY)
The team describes the capability of real-time imaging of electrowetting behavior of Co-based oxide catalysts, the evolution of solid-liquid interfaces and surface degradation, and characterization of gaseous products under alkaline OER. Degradation mechanisms of Pt nanoparticles under cathodic ORR are discussed in the context of beam effects and evolution of cathodic layers over many cycles. Finally, cycle-dependence of the restructuring and dissolution mechanisms of Cu nanospheres under CO2ER and the degradation at highly negative potentials are discussed. The account demonstrates the exceptional potential for in-situ electrocatalyst structural characterization under ec-LPTEM and the advantages of the favorable electrode geometries, high-cycling, and extended potential range enabled by bulk off-chip reference and counter electrodes.
Reference: Tzu-Hsien Shen, Robin Girod, Vasiliki Tileli, Accounts of Chemical Research 56 (21) 3023-3032 (2023) DOI: 10.1021/acs.accounts.3c00463
Full paper Copyright © 2023 The Authors. Published by American Chemical Society. This publication is licensed under CC-BY 4.0.
View All News