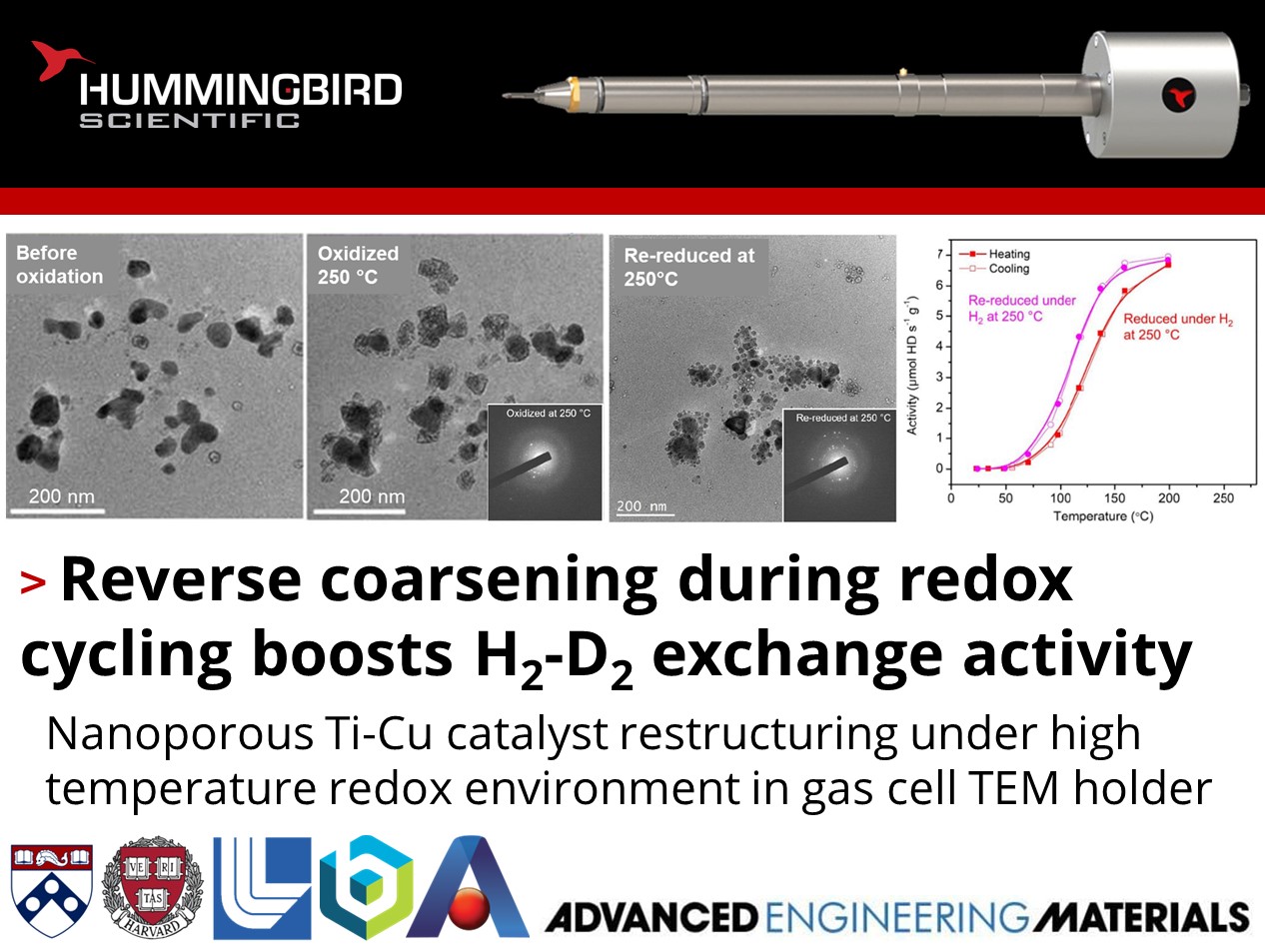
How can redox cycling improve the activity of catalysts?
Alexandre Foucher, Eric Stach, and their colleagues at the University of Pennsylvania, Harvard University, Lawrence Livermore National Laboratory, Brookhaven National Laboratory, and Ames National Laboratory published work using their Hummingbird Scientific in-situ gas heating TEM sample holder to investigate morphological changes and catalytic enhancement of nanoporous Ti-Cu catalysts under reductive H2 and oxidative O2 environments at elevated temperatures.
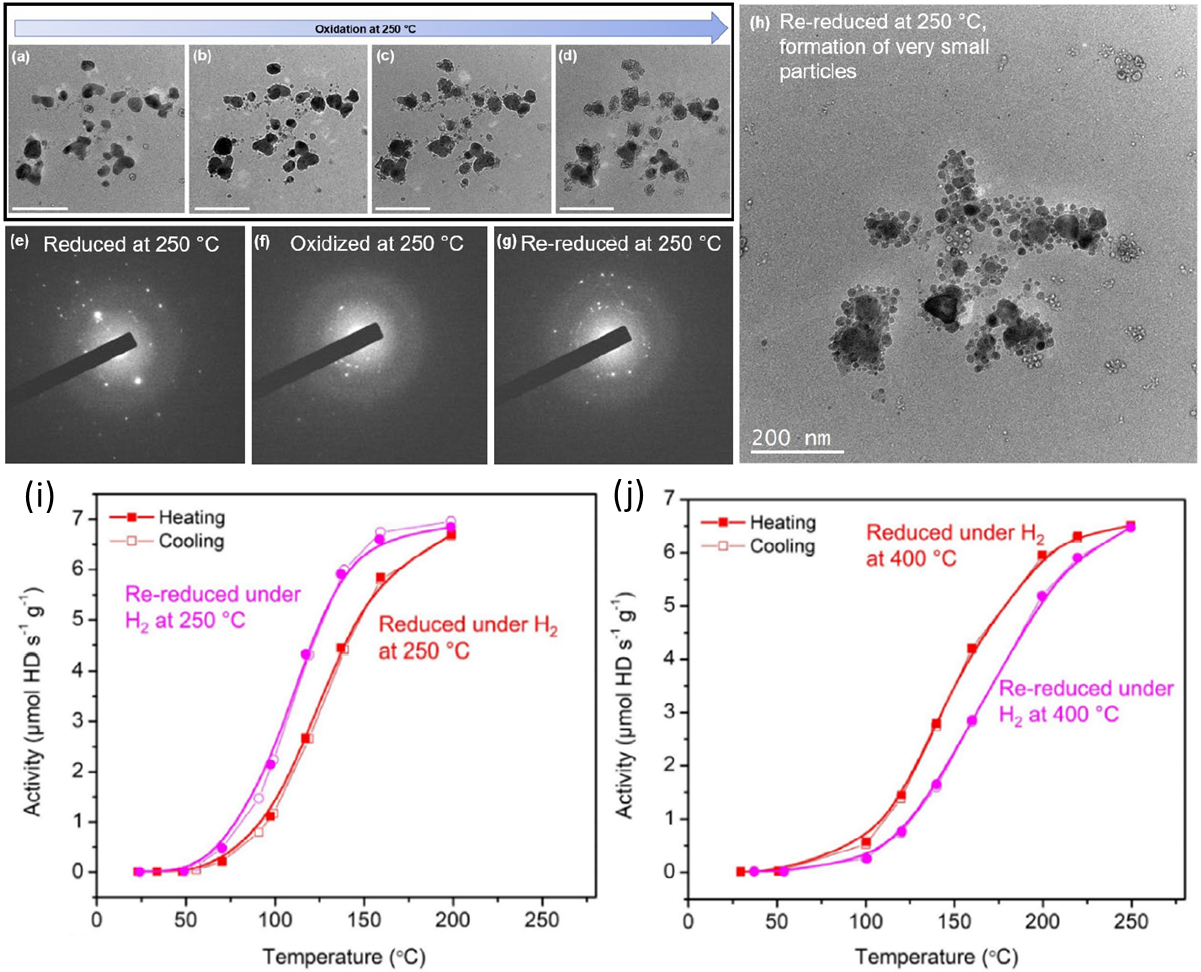
Progressive oxidation of isolated Cu particles and ligaments detached from the main nanoporous structure, scale bars in (a–d) = 200 nm. a–d) Bright-field transmission electron microscopy (TEM) images collected during the oxidation. Diffraction pattern from the group of Cu particles after e) reduction under H2 at 250 °C, f) oxidation at 250 °C, a reduction of the first ring diameter indicates the transformation of metallic Cu to a CuO structure, and g) after re-reduction. Diffraction spots are less intense and more numerous, indicting smaller grain size. h) Corresponding TEM image, showing the formation of very small particles. Hydrogen–deuterium (H2–D2) activity of npTiCu measured under i) H2 at 250 °C and followed by a redox cycle (H2–O2–H2 pretreatments at 250 °C) and j) under H2 at 400 °C and followed by a redox cycle (H2–O2–H2 pretreatments at 400 °C) Copyright © 2023 John Wiley & Sons, Inc.
In situ scanning transmission electron microscopy (STEM) showed that oxidation at 400°C caused expansion and coarsening of the nanoporous ligaments, while subsequent reduction led to particle formation and loss of surface area. A redox cycle at 250°C resulted in fragmentation of the nanoporous structure into smaller particles, increasing the surface area for catalysis. Catalytic testing revealed that the 400°C redox cycle decreased activity due to loss of surface area from particle growth while the 250°C cycle boosted the H2-D2 exchange activity due to increased surface area from fragmentation exposing more active sites. X-ray photoelectron spectroscopy (XPS) showed the titanium surface concentration did not change dramatically with redox cycling temperature, suggesting the activity changes were primarily driven by morphological changes impacting surface area. The results demonstrate a strategy to regenerate and improve the catalytic activity of nanoporous metal catalysts by careful redox treatments to restructure the material into higher surface area states.
Reference:
Alexandre C. Foucher, Jennifer D. Lee, Zhen Qi, Gengnan Li, Gaoyuan Ouyang, Jun Cui, Jorge Anibal Boscoboinik, Cynthia M. Friend, Juergen Biener, and Eric A. Stach, Advanced Engineering Materials 25 (9) 2201724 (2022) DOI: 10.1002/adem.202201724
Full paper Copyright © 2023 John Wiley & Sons, Inc.
View All News