How do electrocatalyst surfaces evolve during reaction with Fe impurities in electrolyte?
Fengli Yang, See Wee Chee, and their colleagues at the Fritz-Haber-Institut der Max-Planck-Gesellschaft published recent work demonstrating the powerful capability of the Hummingbird Scientific Gen V In-situ Bulk Liquid-electrochemistry TEM sample holder to characterize the evolution of octahedral NiO catalyst particles under repeated high-cycle electrochemical cycling in a liquid cell infused with Fe-containing electrolyte. The team complemented in-situ and ex-situ scanning transmission electron microscopy and energy dispersive X-ray spectroscopy (STEM-EDS) with X-ray photoelectron spectroscopy (XPS), time-resolved inductively coupled plasma mass spectroscopy (ICP-MS), operando Raman spectroscopy and X-ray absorption spectroscopy (XAS).
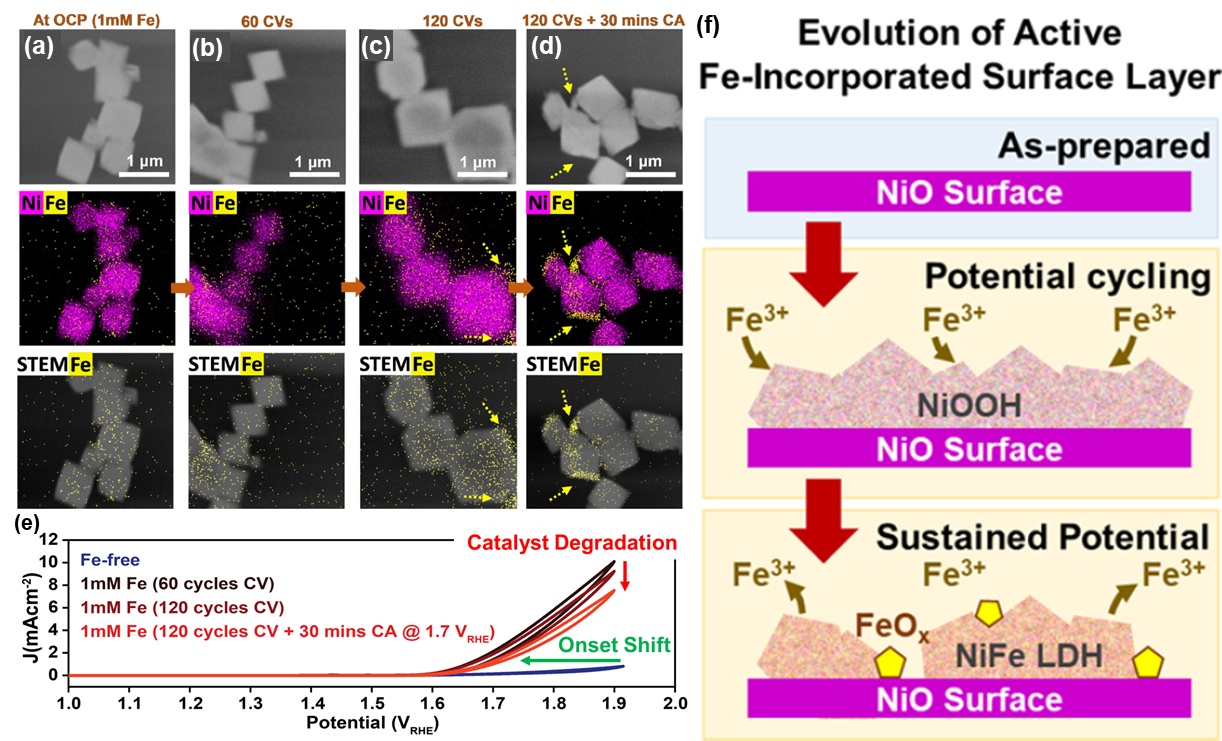
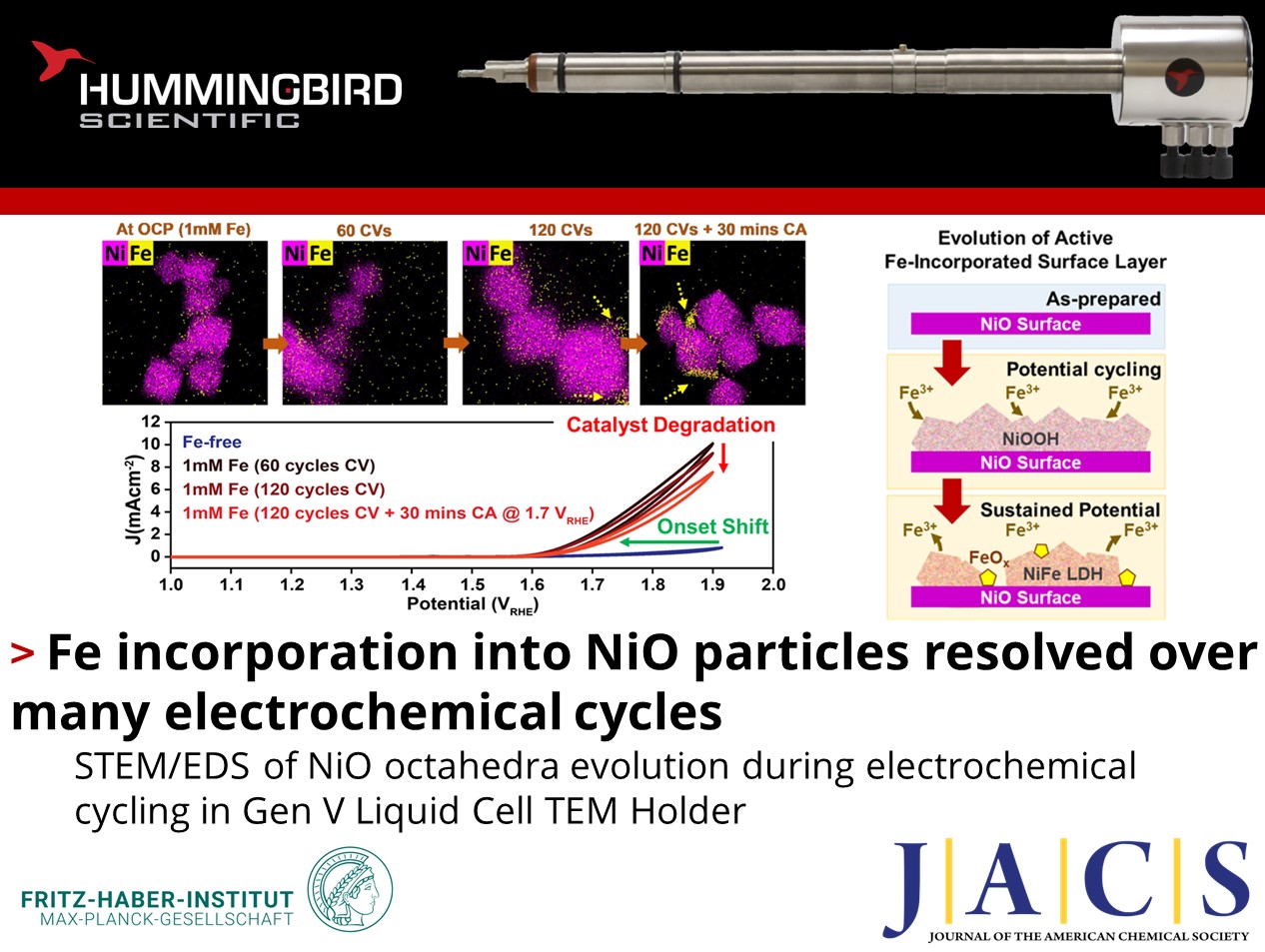
a-d) In-situ STEM images, combined Ni/Fe chemical EDS maps and STEM/Fe EDS maps of NiO octahedra: a) at open circuit potential; b) after 60 cycles of CV, c) after 120 cycles of CV from 0.7 to 1.9 VRHE and d) after 30 more minutes of chronoamperometry at 1.7 VRHE. e) Cyclic voltammogram collected in a borate buffer without Fe to compared to those obtained at 60 and 120 cycles after Fe(NO3)3 was added into the electrolyte, and after 30 more minutes of chronoamperometry. f) Schematic of two-stage mechanism for Fe incorporation into NiO octahedra. Copyright © 2023 The Authors. Published by American Chemical Society.
Particularly, the team acquired time-resolved EDS maps from catalysts under oxygen evolution reaction (OER) conditions with liquid in the cell and applied potential. The maps show that the surface of the NiO particles first enriches with Fe during cyclic voltammetry, but this enrichment eventually saturates leading to Fe aggregates forming with further cycling. The incorporated Fe also depletes from the catalyst surface under sustained potential. More importantly, the electrochemical response for the holder re-produces the expected shift in OER onset potential due to Fe impurities in the electrolyte changing the chemistry of the catalyst, demonstrating the robust electrochemical performance of our Bulk Liquid-electrochemistry holder. Together with the complementary measurements, these results clarify the structural evolution of a NiO surface during OER and the critical role of Ni-Fe layered double hydroxide formation and degradation in determining the electrocatalytic performance.
Reference:
Fengli Yang, Mauricio Lopez Luna, Felix T. Haase, Daniel Escalera-López, Aram Yoon, Martina Rüscher, Clara Rettenmaier, Hyo Sang Jeon, Eduardo Ortega, Janis Timoshenko, Arno Bergmann, See Wee Chee, and Beatriz Roldan Cuenya, J. Am. Chem. Soc. 145 (39) 21465-21474 (2023) DOI: 10.1021/jacs.3c07158
Full paper Copyright © 2023 The Authors. Published by American Chemical Society.
View All News