Using in-situ and ex-situ electron microscopy, researchers at the Department of Interface Science, Fritz-Haber Institute of the Max Planck Society have presented a realistic picture of how Cu2O catalysts dynamically re-structure under CO2RR reaction conditions.
Hummingbird Scientific’s Generation V Liquid Electrochemistry TEM holder was used to recreate the bulk experiment in-situ.
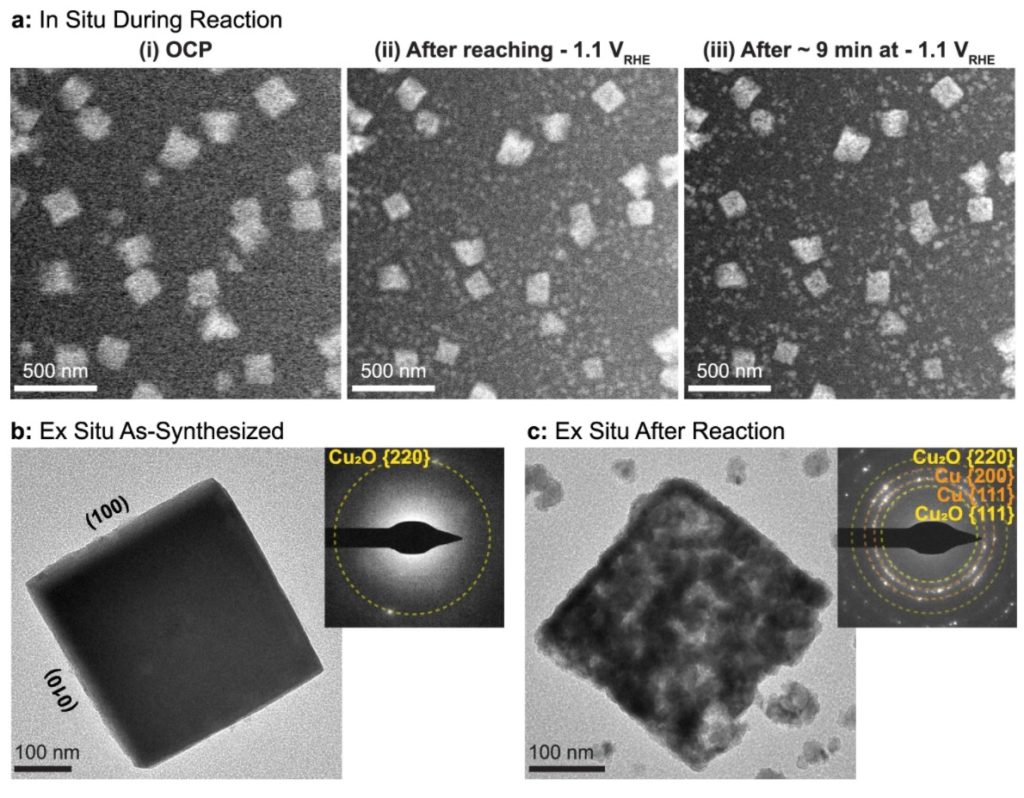
a Sequence of images illustrating the morphological changes observed in ~170 nm cubes after (i) introducing CO2 saturated 0.1 M KHCO3 under open circuit potential, (ii) while applying a reductive potential of −1.1 VRHE and (iii) after ~9 min at −1.1 VRHE, where a large bubble formed. The image sequences were all acquired with an electron flux of 1.7 e− Å−2 s−1. Comparison of Cu2O cubes b before and c after reaction using ex-situ TEM imaging and electron diffraction (upper right inserts). Copyright © 2021 Nature Communications
In this work, researchers followed the morphological evolution of Cu2O cubes during CO2 electroreduction, demonstrating their complexity under reaction conditions, observing detachment, fragmentation, and re-deposition processes.
Reference: Philipp Grosse, Aram Yoon, Clara Rettenmaier, Antonia Herzog, See Wee Chee & Beatriz Roldan Cuenya “Dynamic transformation of cubic copper catalysts during CO2 electroreduction and its impact on catalytic selectivity” © 2021 Nature Communications. Full Paper
View All News