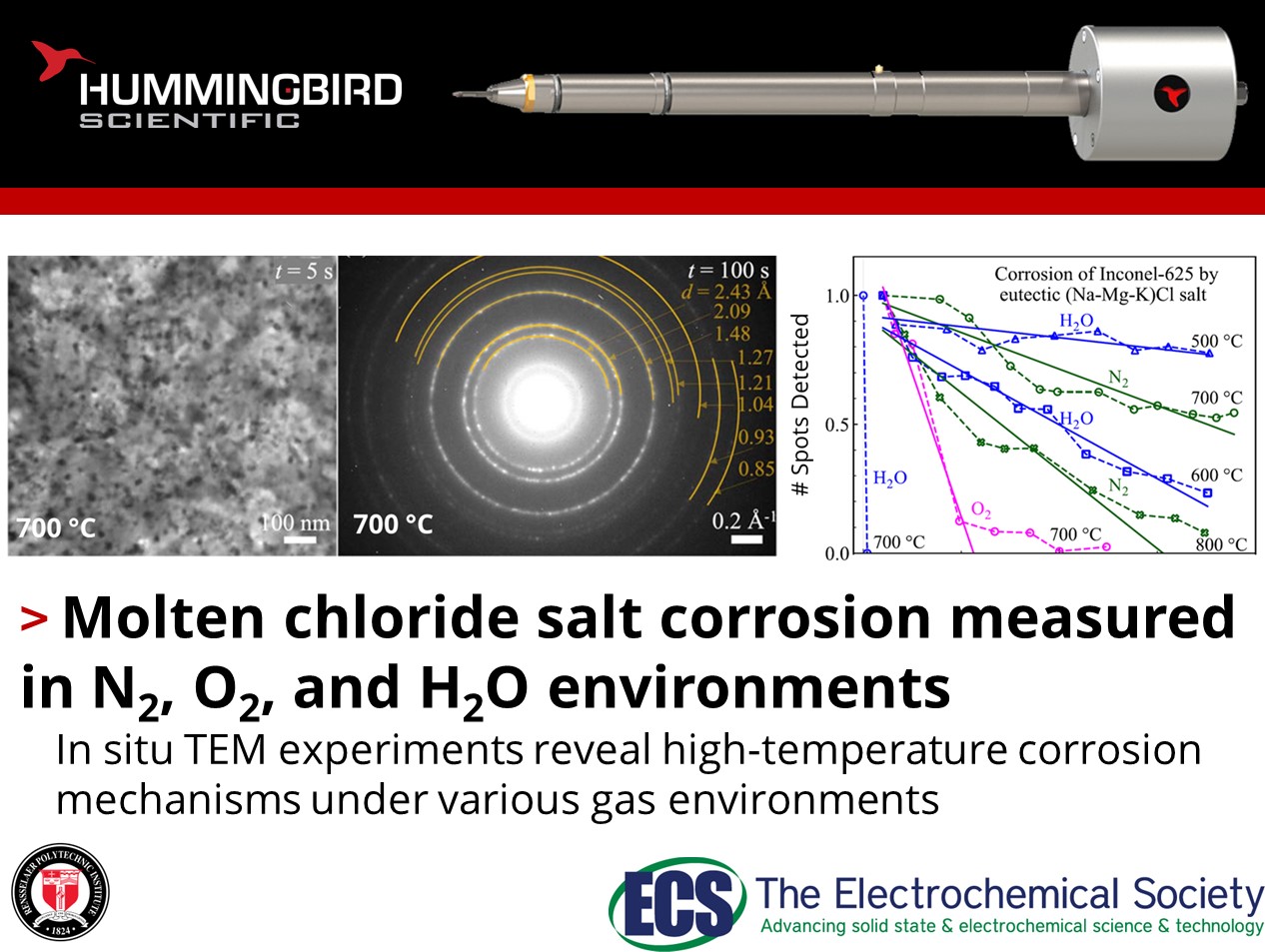
How does environment impact molten chloride salt corrosion in Ni-based alloys?
Prachi Pragnya, Daniel Gall, and Robert Hull from Rensselaer Polytechnic Institute published an exciting study using their Hummingbird Scientific in-situ TEM single-channel gas-environmental heating sample holder to measure the temperature dependence of molten chloride salt corrosion in Inconel-625 under three environmental conditions: inert N2, oxidizing O2, and controllably hydrated under high vacuum.
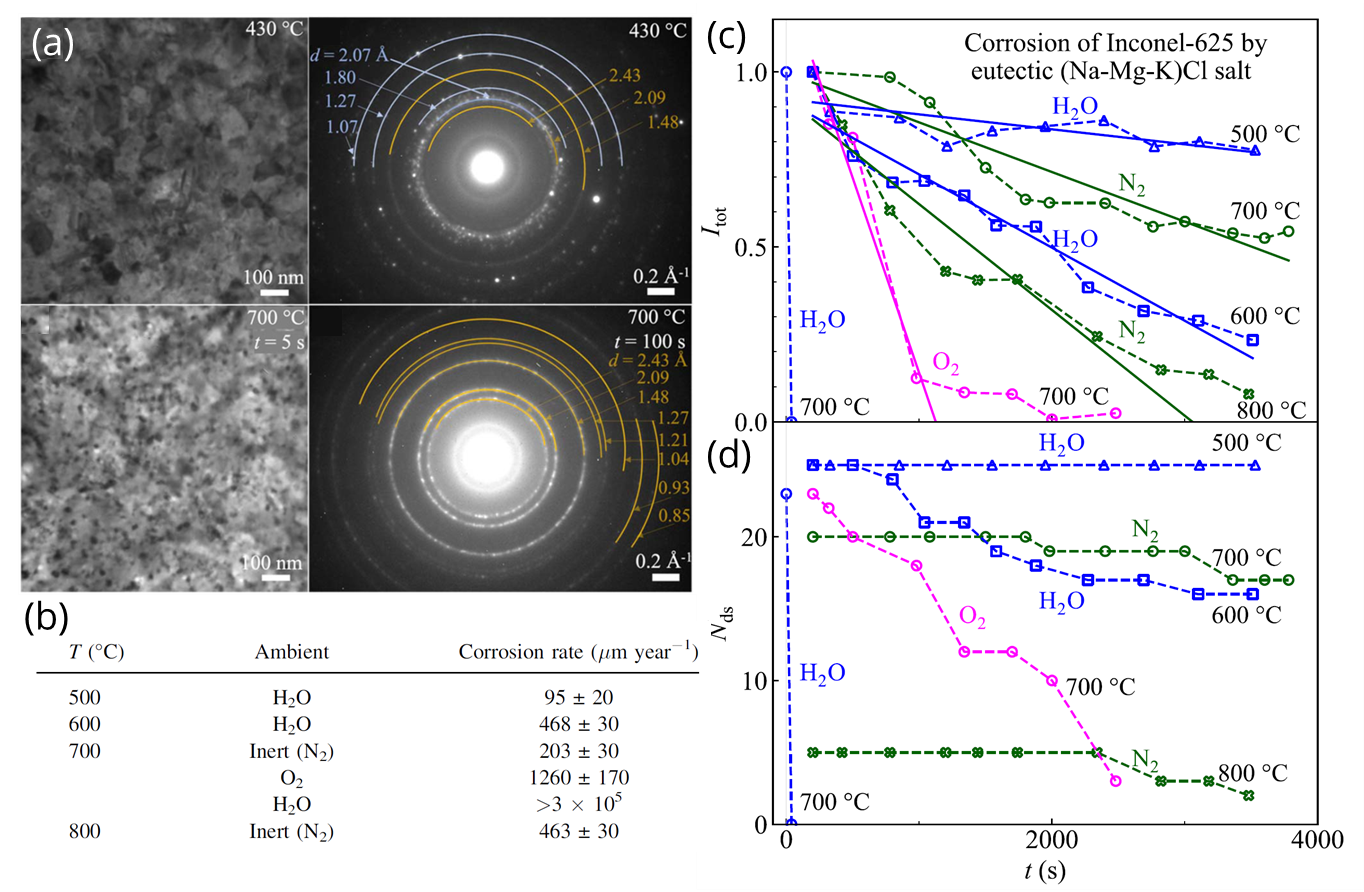
Figures showing a) situ TEM-EC micrographs and diffraction patterns from a 50 nm thick Inconel-625 layer in contact with hydrated chloride salt, collected at 430 °C (top) and 700 °C (bottom). b) Corrosion rate table for all six environment/temperature combinations. c) Normalized diffraction pattern total intensity Itot and d) number of detected diffraction spots vs corrosion time t. Copyright © 2022 The Electrochemical Society
The corrosion rate was measured by analyzing in situ diffraction patterns taken during exposure, and was found to increase with temperature across all environmental conditions. At 700 °C, O2 ambient led to a six-fold increase in corrosion rate over inert N2, while salt hydration led to an increase of three orders of magnitude which was drastically reduced at 600 °C and below. Compositional analysis was performed ex-situ using X-ray photo-electron spectroscopy (XPS) and Auger electron spectroscopy (AES), and indicated that corrosion on O2 is driven by oxidation of metals by dissolved O2 gas, while corrosion in hydrated salts was dominated chlorination of metals by dissolved HCl gas and MgOH+ ions. They concluded that salts with low solubility for O2 and HCl will be more resistant to corrosion at elevated temperature. The work demonstrates the utility of the Hummingbird Scientific single-channel gas-environmental heating TEM sample holder for assessing the effect of various environments and impurities on molten salt corrosion rates.
Reference: Prachi Pragnya, Daniel Gall, and Robert Hull, Journal of the Electrochemical Society 169 (37), 093504 DOI: 10.1149/1945-7111/ac8376
Full paper Copyright © 2022 The Electrochemical Society
View All News