How does the X-ray liquid electrochemistry holder expose the role of cationic species in electrocatalyst selectivity?
Hummingbird Scientific has collaborated with Juwon Kim, Jongwoo Lim, and their colleagues at Seoul National University, the Institute for Basic Science, Korea Advanced Institute of Science and Technology, Chungbuk National University, Korea Institute of Science and Technology, Korea University of Science and Technology, Berkeley Lab, the National Synchrotron Research Radiation Research Center, to publish recent work tracking cationic Cu species during CO2 electrocatalysis under reductive conditions with the Hummingbird Scientific Generation V bulk liquid-electrochemical scanning transmission X-ray microscopy (STXM) system. Phase changes and selectivity of hydroxide-derived Cu electrocatalysts were characterized using operando soft X-ray microscopy and mechanistic insights were supported by density functional theory (DFT) calculations.
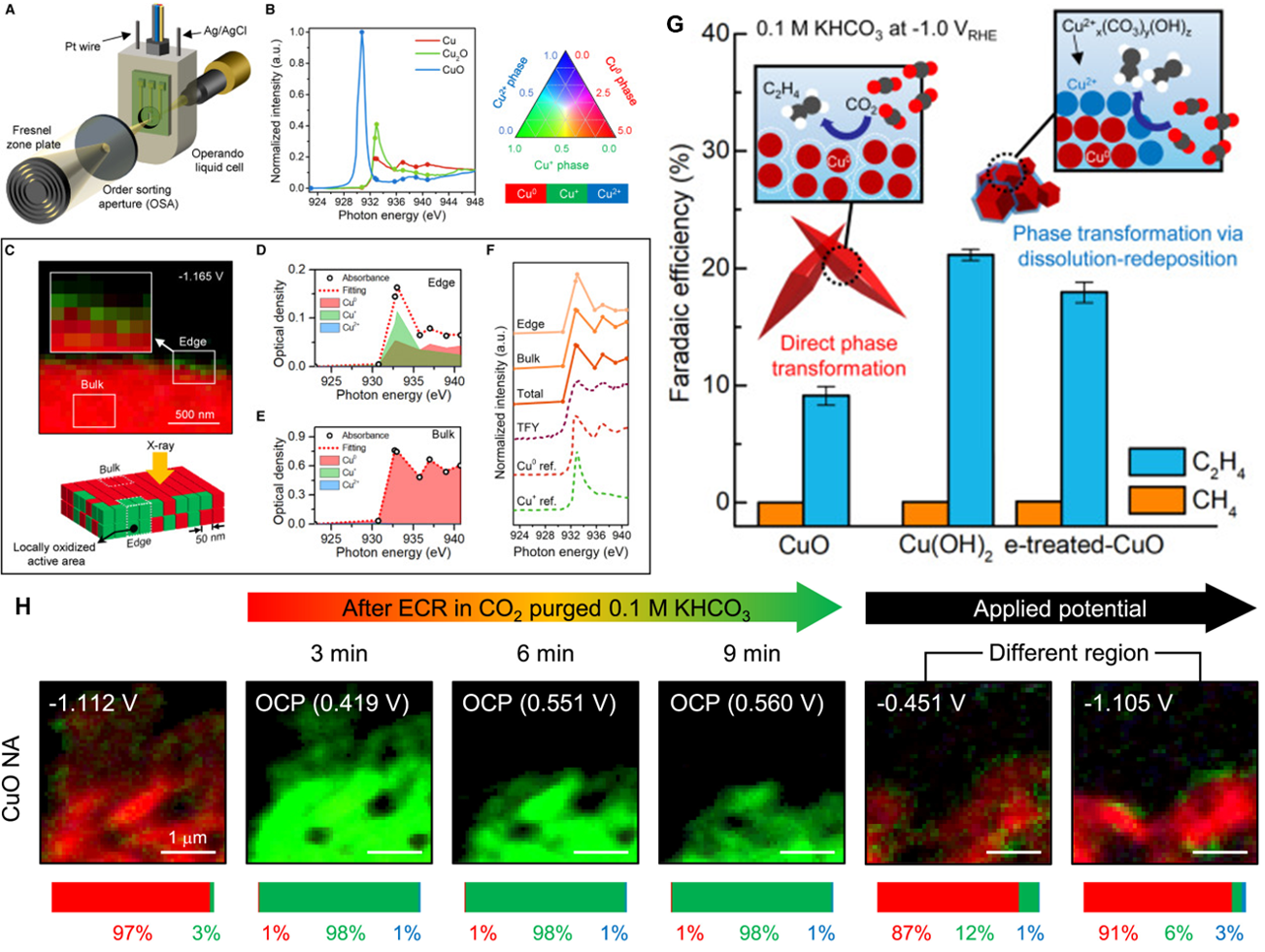
a) Schematic illustration of operando STXM characterization system. b) Cu L3-edge absorption spectra of Cu metal, Cu2O, and CuO and ternary phase diagram. c) Chemical mapping at -1.165 VRHE and optical density in the d) edge and e) bulk regions of (c). f) Comparison of absorption spectra measured using STXM and total fluorescence yield (TFY). g) Faradic efficiencies showing increased efficiency for C-C coupling for Cu(OH)2 and e-treated CuO, with inset schematic illustrations of phase transformation mechanisms. h) Chemical composition maps of CuO NA after ECR in CO2-saturated 0.1 M KHCO3 at open circuit potential and under applied potential. Copyright © 2024 Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Using real-time observation of Cu oxidation states and morphological evolution of the electrochemical CO2 reduction (ECR) catalysts, the mechanisms governing the dynamic transformation of cationic Cu species and corresponding enhancement to performance were determined. Cu2+ phases such as copper(II)-carbonate-hydroxide species boosted C-C coupling, and may dynamically persist under a cathodic environment. While observing the formation of surface Cu2+ species, the team was able to electrochemically redirect less active catalysts into more highly active catalysts for C-C coupling using applied potential. The approach and hardware provide new insights into electrocatalysis mechanisms and will inform future design of advanced electrocatalysts.
Reference:
Juwon Kim, Si Young Lee, Se-Jun Kim, Bonho Koo, Jinkyu Chung, Danwon Lee, Subin Choi, Jimin Kim, Sungjae Seo, Chihyun Nam, Karl Adrian Gandionco, Gwangsu Bak, Sugeun Jo, Namdong Kim, Hyun-Joon Shin, Keun Hwa Chae, Da Hye Won, Matthew A. Marcus, David A. Shapiro, Shu-Chih Haw, Daan Hein Alsem, Norman J. Salmon, Byoung Koun Min, Hyungjun Kim, Yun Jeong Hwang, Jongwoo Lim Joule 8 1-13 (2024) DOI: 10.1016/j.joule.2024.09.008
Full paper Copyright © 2024 Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
View All News

