How do copper and iridium alloy to form efficient and stable catalyst nanoparticles?
Alexandre Foucher, Eric Stach, and their colleagues at the University of Pennsylvania, Paul Scherrer Institute, and Stony Brook University published recent work characterizing Ir nanoshells derived from core-shell Cu-Ir catalyst nanoparticles with the Hummingbird Scientific gas heating TEM sample holder. In-situ high-temperature oxygen reduction and oxygen evolution reactions under scanning transmission electron microscopy with energy dispersive X-ray spectroscopy (STEM-EDS) were performed alongside X-ray absorption spectroscopy (XAS) to investigate the stability and evolution of nanoparticle structure and chemistry under working conditions.
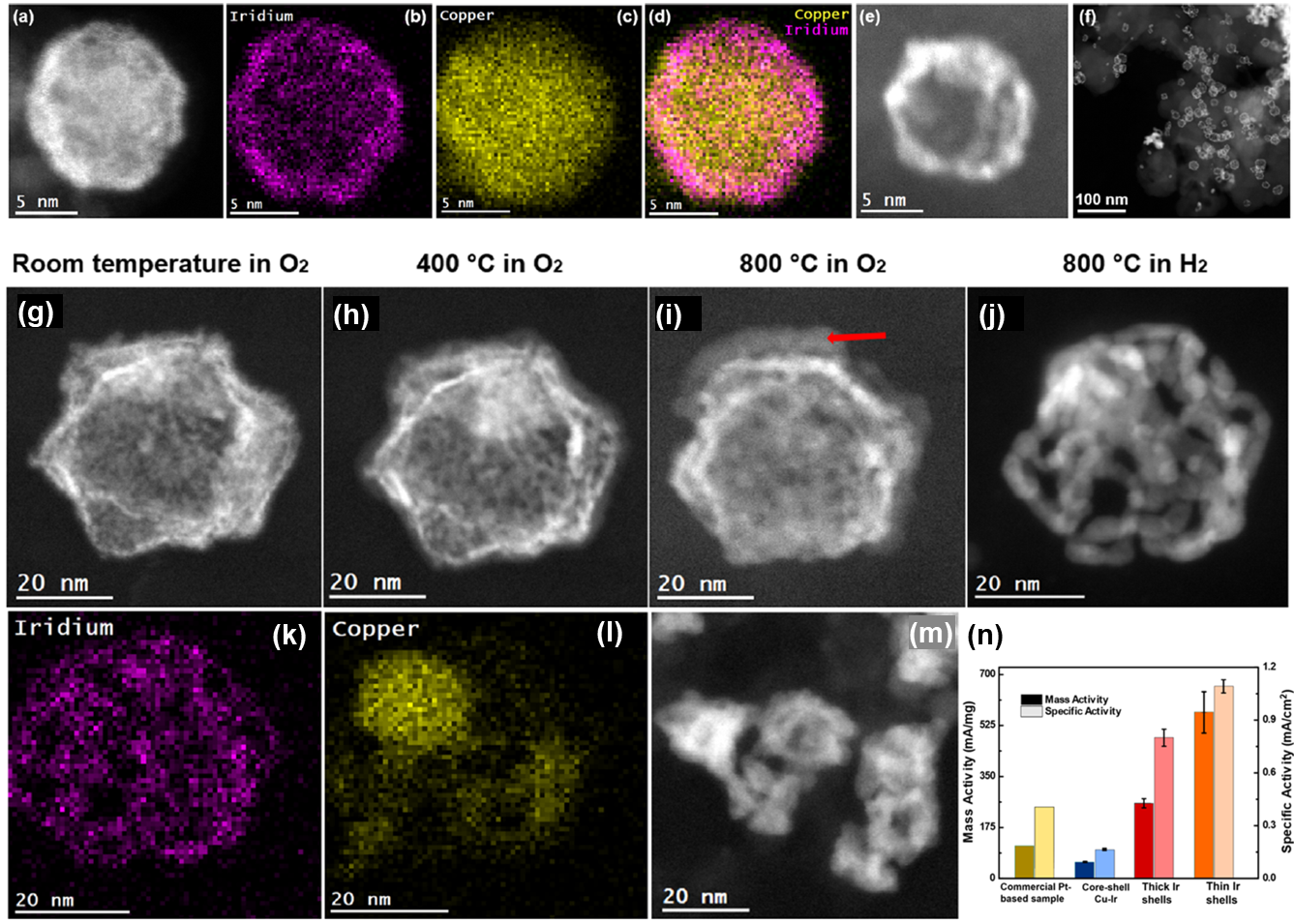
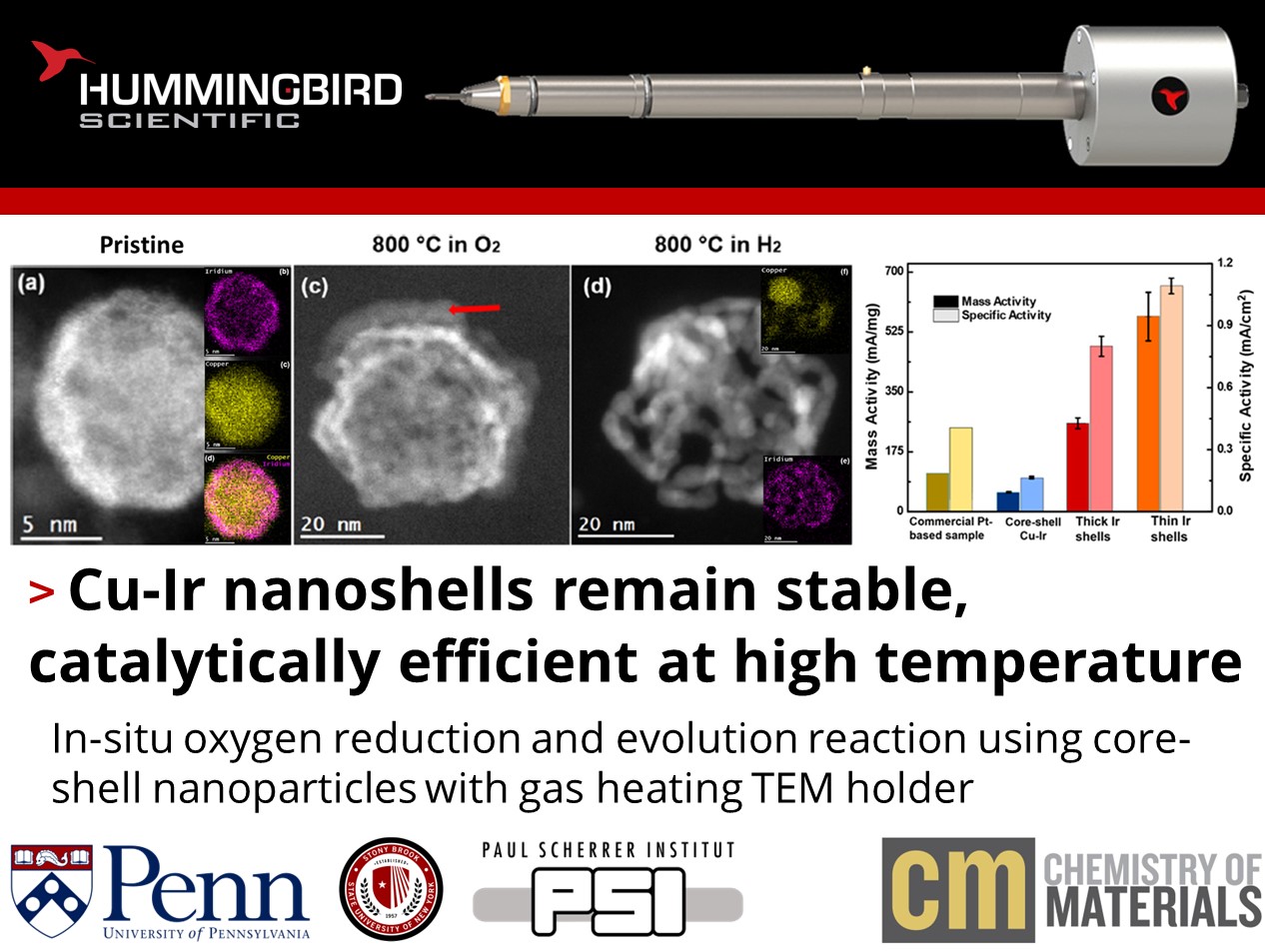
a) HAADF-STEM image of a freshly synthesized Cu0.79Ir0.21 particles with a core−shell configuration. b) EDS map for Ir. c) EDS map for Cu. d) Combined EDS data. e) Empty shell of another particle after calcination. The void left by the copper after oxidation is clearly visible. f) Large area micrograph showing empty Ir shells on C support. In situ STEM analysis of a Cu0.79Ir0.21 particles with 1 bar of gases. g) Fresh sample. Oxidation in air caused some removal of the Cu core. h) After 30 min exposure to O2 at 400 °C. i) After 30 min exposure to O2 at 800 °C. The red arrow indicates Cu oxide. j) After 20 min exposure to H2 to reduce the Cu and make the Ir shell more visible. k, l) Corresponding EDS maps to the image in (j). m) Other particles after the same treatment. The spherical shape was maintained and did not collapse. n) Mass and specific activities for the I-shells, Cu-Ir nanoparticles, and commercial Pt-based catalysts. Copyright © 2023 American Chemical Society
In-situ gas heating experiments were performed in the Hummingbird gas holder under O2 and H2 environments up to 800 °C, where the remarkable stability of the thick Ir-shells was demonstrated. The thick and thin Ir shells outperformed both the core-shell Cu-Ir particles as well as a commercially available Pt-based catalyst. XAS revealed residual Cu remaining inside the shells after oxidation, which help to rationalize the trend in catalytic activity. The multimodal approach to nanoparticle characterization revealed design principles for future optimization of the stability and performance of nanocatalysts, reducing precious metal usage and cost.
Reference:
Alexandre C. Foucher, Daniel J. Rosen, Shengsong Yang, Dario Ferreira Sanchez, Ilia Sadykov, Daniel Grolimund, Anatoly I. Frenkel, Christopher B. Murray, and Eric A. Stach, Chem. Mater. 35 (11) 4572-4580 (2023) DOI: 10.1021/acs.chemmater.3c00970
Full paper Copyright © 2023 American Chemical Society
View All News