How does the X-ray bulk liquid electrochemistry holder enable visualization of phase separation pathways during operando cycling of LixFePO4?
Researchers at the Seoul National University, MIT, Canadian Light Source, Pohang Accelerator Laboratory, and the Helmholtz-Zentrum Berlin leveraged the capabilities of the Hummingbird Scientific Generation V in-situ X-ray bulk liquid electrochemistry sample holder to perform operando scanning transmission X-ray microscopy (STXM) of LixFePO4 (LFP) particles during charge/discharge cycles to study lithium transportation mechanisms.
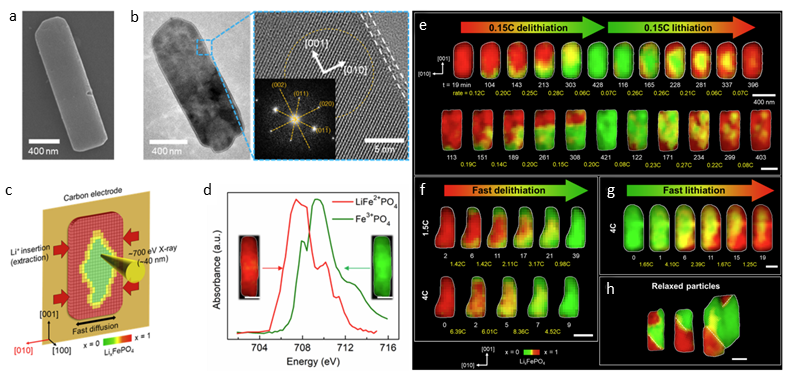
Electron microscopy and operando STXM of [100]-oriented LFP particles (a) SEM image showing the (100) surface of a primary LiFePO4 (LFP) particle. (b) TEM and high-resolution TEM along the [100] zone axis; inset shows FFT of the marked area (orange dashed circle). (c) Schematic of operando STXM setup for LFP/C electrode in EC/DMC electrolyte, using a ~700 eV soft X-ray beam focused to ~40 nm. (d) X-ray absorption spectra at the Fe L₃-edge for LiFePO₄ (Li-rich, red) and FePO₄ (Li-poor, green) phases in electrolyte. (e) Li distribution maps of two particles during (de)lithiation at 0.15C. (f–g) Li maps at faster delithiation rates (1.5C, 4C) and lithiation (4C). Red = Li-rich, green = Li-poor. Time (min) and rate shown below each frame. (h) Ex situ Li maps of particles after 3 months of relaxation. All images aligned with [001] vertical. Scale bar: 400 nm.
The team imaged LFP particles during electrochemical cycling at varying charging rates (C-rates)—0.15C, 1.5C, and 4C. At 0.15C delithiation, lithium exited the particles through specific [010] channels, forming intercalation wave patterns. In contrast, lithiation at the same rate produced a more uniform lithium distribution characterized by solid-solution behavior and a shrinking-core morphology. At higher C-rates, both lithiation and delithiation activated broader surface areas and occurred more concurrently across the particle, leading to diffuse, inward-propagating Li-rich or Li-poor domains. Interestingly, fast delithiation bypassed the orderly, channel-specific mechanism observed at lower rates.
These observations were made possible by the use of the Hummingbird Scientific Generation V in-situ X-ray bulk liquid electrochemistry sample holder. The holder provided a realistic liquid electrochemical environment compatible with spectromicroscopic X-ray imaging, enabling real-time visualization of rate-dependent phase transformations.
The study uncovered a fundamental asymmetry between lithiation and delithiation, governed by differences in surface lithium composition. These findings not only reconcile competing models of lithium transport at various charging rates but also advance our understanding of dynamic processes in lithium-ion battery materials—key to designing faster, more efficient energy storage systems.
Reference: Bonho Koo, Jinkyu Chung, Juwon Kim, Dimitrios Fraggedakis, Sungjae Seo, Chihyun Nam, Danwon Lee, Jeongwoo Han, Sugeun Jo, Hongbo Zhao, Neel Nadkarni, Jian Wang, Namdong Kim, Markus Weigand, Martin Z. Bazant, and Jongwoo Lim, Energy & Environmental Science 16, 3302-3313 (2023). DOI: 10.1039/D3EE00341H
Full paper Copyright © 2023 Royal Society of Chemistry
View All News