In-situ TEM high-temperature reduction of high-entropy alloy nanocatalyst
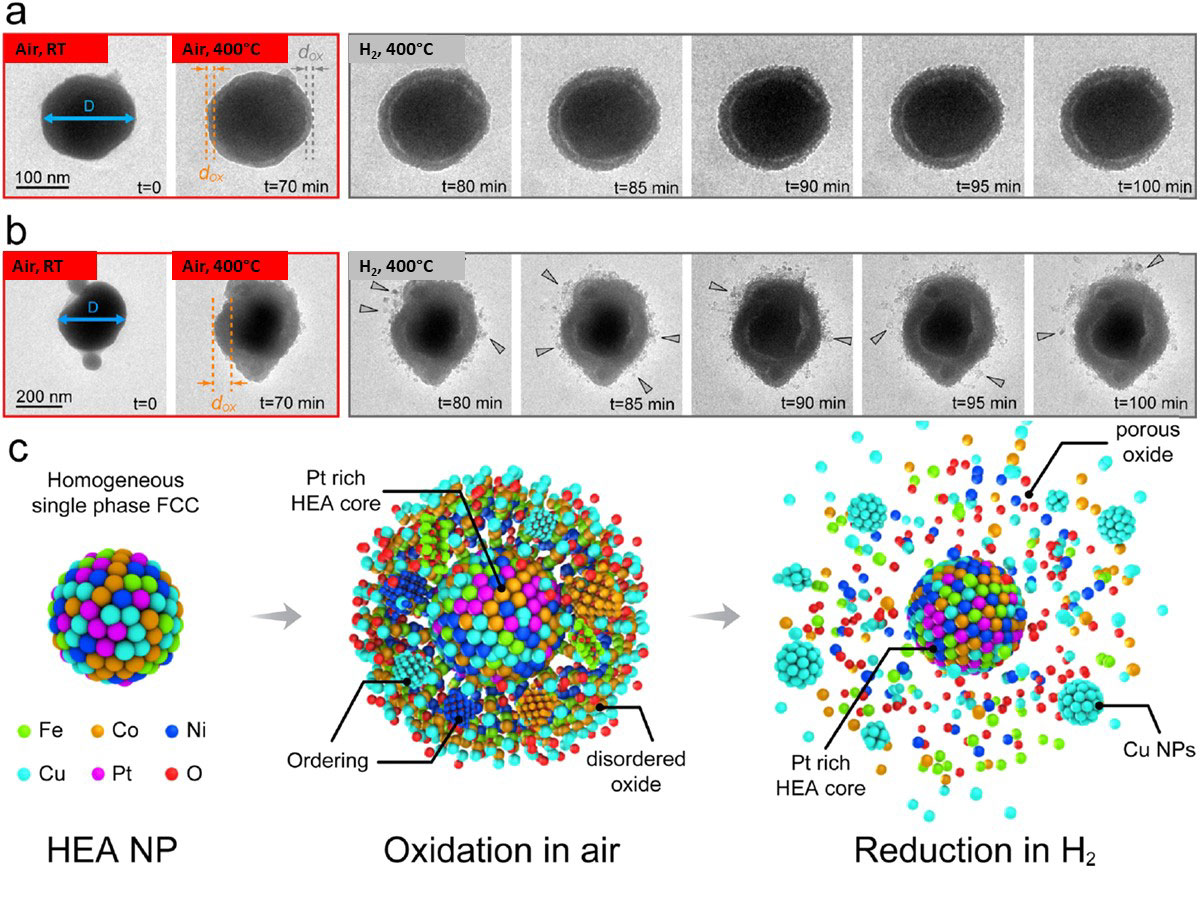
Atomistic interaction of gas-solid phase is important to understand the working mechanism of various catalyst materials. This is specifically the case for high-entropy alloy (HEA) atomistically mixed with more than five elements. A research team from the University of Illinois, Chicago (UIC), Argonne National Laboratory (ANL), University of Pittsburgh, University of California, Riverside, and Northwestern University used Hummingbird Gas TEM holder to study the oxidation and reduction behavior of FeCoNiCuPt HEA in air and hydrogen gas at 400 °C, respectively. As the particles are heated in air at 400 °C, there is a growth of the oxide layer around the particles. Upon introduction of hydrogen gas, there is a further expansion of the oxide layer which transforms into porous structures and there is an outward diffusion of all transition metals (Fe, Co, Ni and Cu). The work presented here provides fundamental insights into the new class of alloy NPs for catalytic applications.
Figure: In-situ TEM oxidation and reduction of CoNiCuPt nanoparticles upon heating at 400 °C, in air and hydrogen, respectively. A shown in the schematic, all transition metals diffuse out. Pt remains intact in the core.
Image Copyright © 2021 American Chemical Society
Reference: Song et al. Nano Lett. 2021, 21, 4, 1742–1748. DOI: 10.1021/acs.nanolett.0c04572