How do copper and platinum alloy as efficient and stable catalyst nanoparticles?
Alexandre Foucher, Eric Stach, and their colleagues at the University of Pennsylvania, Harvard University, Paul Scherrer Institute, and Stony Brook University published recent work characterizing newly synthesized Cu-Pt catalyst nanoparticles with the Hummingbird Scientific gas heating TEM sample holder. In-situ high-temperature annealing, gas treatments under STEM and EDS/EELS, in-situ X-ray absorption spectroscopy (XAS), and CO oxidation measurements were conducted to characterize the nanoparticle microstructural evolution under redox conditions and catalytic activity.
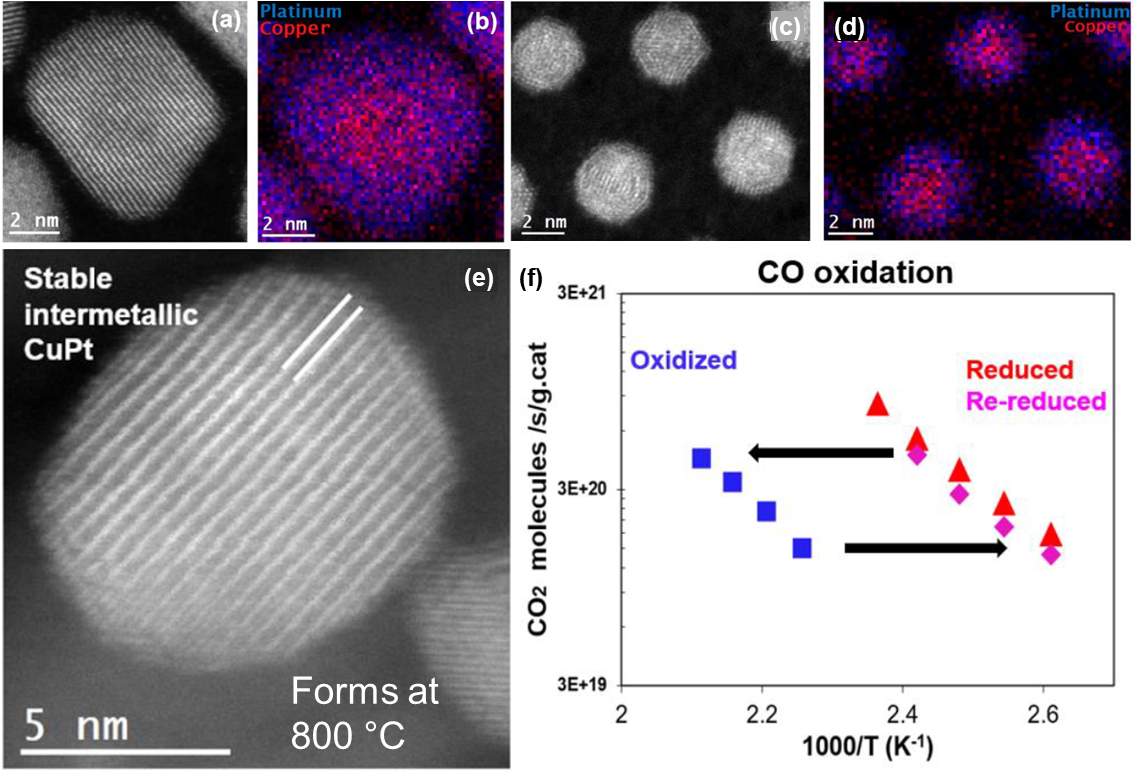
STEM-EELS and EDS analysis of the fresh large and small nanoparticles. a) HAADF-STEM image of a large Cu-Pt nanoparticle. b) Combined EDS maps for Cu and Pt showing a Cu-rich core and enveloped in a thick Pt-rich shell. c) HAADF-STEM image of small Cu-Pt nanoparticles. d) Combined EDS maps for Cu and Pt showing a Cu-rich core and enveloped in a Pt-rich shell. e) HAADF-STEM image of large nanoparticle after heat treatment and conversion into a intermetallic. f) Reduction, oxidation, and re-reduction of large Cu-Pt particles showing recovery of activity and temperature dependence. Copyright © 2023 American Chemical Society
The as-synthesized nanoparticles have Cu-rich cores and Pt-rich surfaces and came in large (9 nm) and small (3 nm) sizes depending on synthesis procedure. Fresh particles were stable under redox conditions at 400 °C, with the Pt-rich layer protecting the Cu core from oxidation. After annealing at 800 °C, the particles transformed into an intermetallic CuPt phase with O2 oxidation fully reversible by H2 reduction. Higher activity was measured for CO oxidation than pure Pt nanoparticles at a lower temperature and with lower overall surface area. The demonstrated stability and reversibility of catalytic degradation of CuPt nanostructures is relevant to other chemical processes and may help to reduce the cost of catalysts by reducing necessary Pt content.
Reference:
Alexandre C. Foucher, Shengsong Yang, Daniel J. Rosen, Renjing Huang, Jun Beom Pyo, Ohhun Kwon, Cameron J. Owen, Dario Ferreira Sanchez, Ilia Sadykov, Daniel Grolimund, Boris Kozinsky , Anatoly I. Frenkel, Raymond J. Gorte, Christopher B. Murray, Eric A. Stach, J. Am. Chem. Soc. 145 (9) 5410-5421 (2023) DOI: 10.1021/jacs.2c13666
Full paper Copyright © 2023 American Chemical Society
View All News