Researchers from the University of Pennsylvania, Stony Brook University, Harvard University, and Brookhaven National Laboratory conducted an in situ TEM experiments using Hummingbird Scientific’s in-situ TEM Gas heating holder system to characterize NiCu3 nanoparticles with in situ scanning transmission electron microscopy and electron energy-loss spectroscopy (STEM-EELS). They then ran the same experiments using in situ X-ray absorption spectroscopy (XAS) and overlaid the results. From those experiments they mapped the dynamical restructuring effects at the atomic scale of these catalysts during in-situ oxidation and reduction.
With Improved high resolution and accuracy EDS mapping with less background at the highest temperatures, Hummingbird Scientific’s in-situ TEM Gas Heating holder also minimizes drift to the point that one can collect data without drift-correction. The work presented here using the Gas Heating Cell TEM/X-Ray sample holder shows a unique method to perform controlled monitoring of high-temperature oxidation/reduction processes at ambient conditions across multiple microscope platforms that combine imaging with chemical analysis.
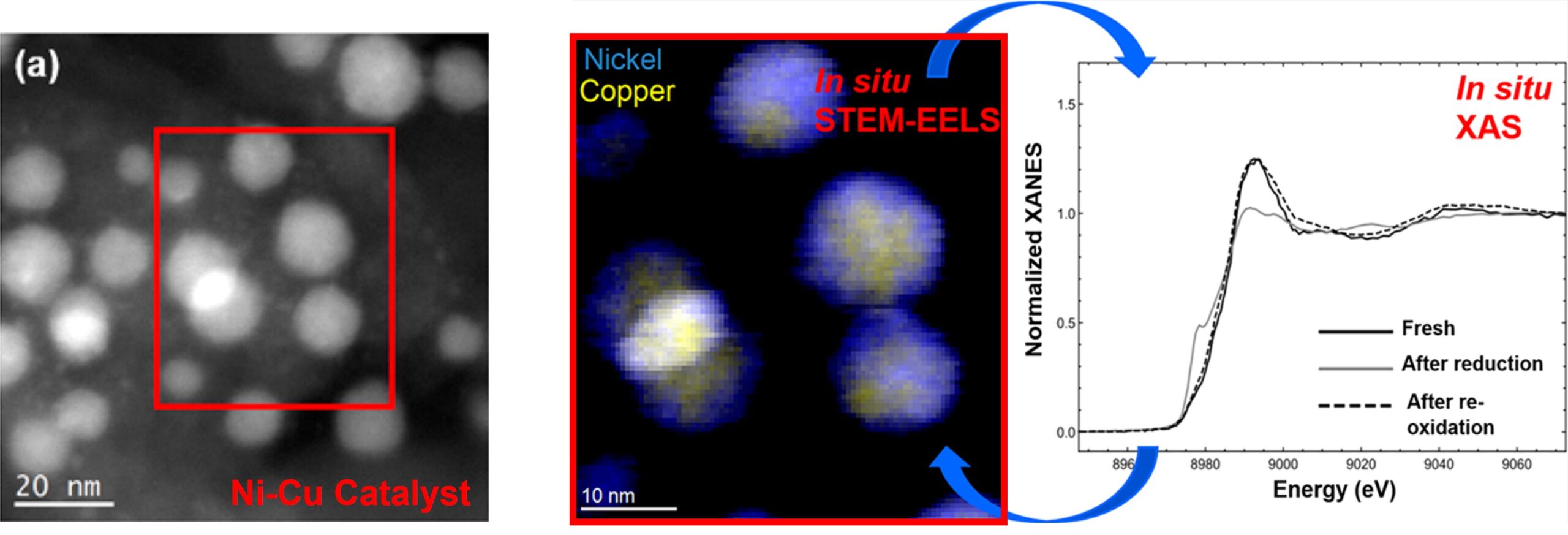
EELS maps particles after reduction under H2 at 400 °C for 2 h. The red box indicates the region mapped with EELS. (Left) Initial core−shell structure after reduction with no visible shell on the HAADF-STEM image. (Middle) EELS maps show the presence of a thin Ni-rich surface. Thus, reduction did not destroy the core−shell configuration. (Right) XANES and Fourier transform (FT)-EXAFS data of the sample, containing the two configurations, during the redox cycle. The Cu K edge was used to determine the coordination numbers of the sample fresh, after reduction, and after reoxidation. Copyright © 2022 American Chemical Society
Reference: Alexandre C. Foucher, Nicholas Marcella, Jennifer D. Lee, Ryan Tappero, Christopher B. Murray, Anatoly I. Frenkel, and Eric A. Stach “Dynamical Change of Valence States and Structure in NiCu3 Nanoparticles during Redox Cycling” J. Phys. Chem. C. Full paper © 2022 American Chemical Society.
View All News