How can proteins be used as a template for biomineral growth?
Fatima A. Davila-Hernandez, James J. De Yoreo, David Baker, and their colleagues at the University of Washington-Seattle and Pacific Northwest National Laboratory published recent work using their Hummingbird Scientific in-situ liquid flow TEM sample holder to investigate the nucleation, growth, and assembly of hierarchically structured calcium carbonate biominerals. The team hypothesized that molecular templates could be used to program biomineral nucleation and growth using de novo protein design.
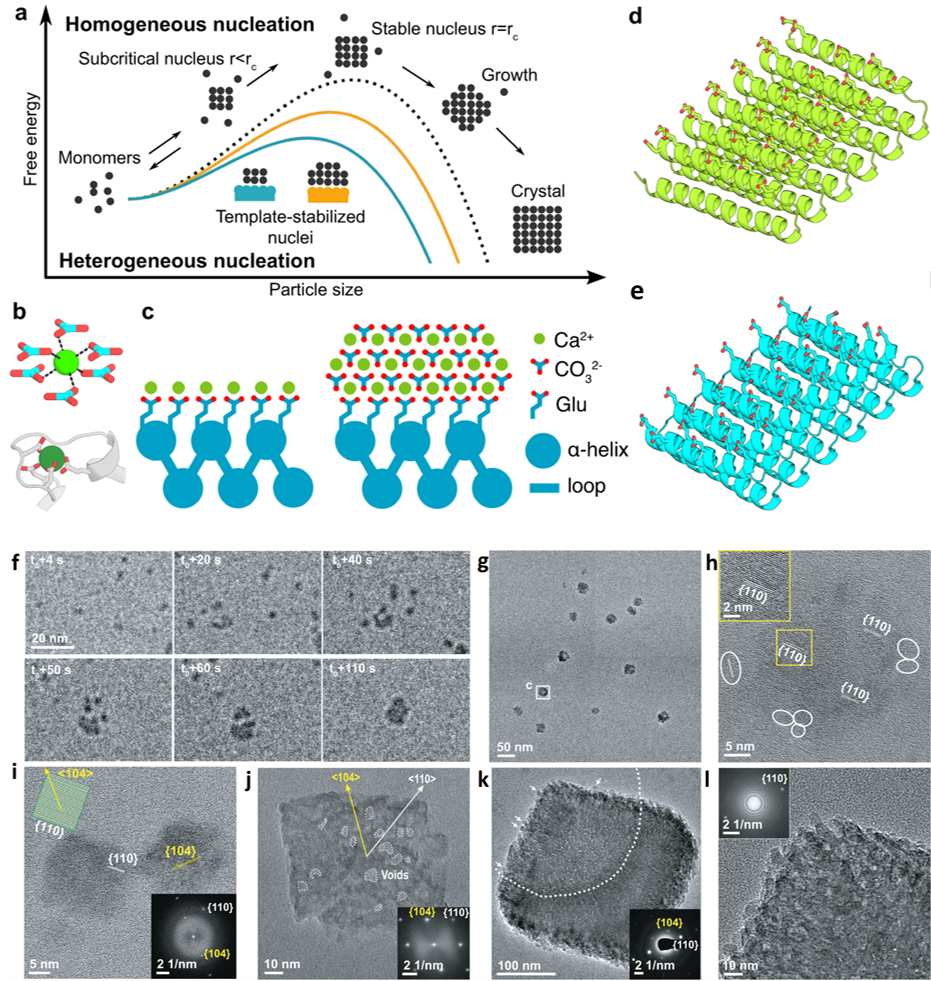
a) Schematic illustrating the reduction of the free energy barrier of nucleation and the critical radius of the nuclei using a template. b) Coordination of calcium (top) by carbonate ions within a unit cell of calcite, and (bottom) by carboxylate containing glutamate and aspartate residues in the structure of calmodulin. c) Tessellating binding moieties across repeated α-helices within a designed protein capable of pre-organizing bound calcium ions. d–e)Illustration of the designed proteins. f) Sequential LP-TEM image sequence shows the attachment of multiple calcite nanocrystals into a larger structure. g) Ex situ TEM image showing calcite grown through particle attachment. Mean particle size is 22.8 ± 3.9 nm. h) HR-TEM image showing the aggregated crystal with {110} lattice orientation. White frames mark several individual calcite nanocrystals around the larger particle. i) HR-TEM image showing additional calcite nanocrystals with a size of ≈ 20 nm and {110} side faces with inset showing the calcite morphology and facet information. j) TEM image showing ≈ 100 nm rhombohedral calcite with inset FTT shows the crystal orientation and single crystallinity. k) TEM image showing larger rhombohedral calcite with embedded SAED image demonstrating its single crystalline nature. Arrows mark several calcite nanocrystals on the surface. i) HR-TEM and corresponding FFT images confirming the single crystalline nature and that the rough surface is composed of attached nanoparticles. Copyright 2024 Springer Nature Limited
Under in-situ TEM observation, designed helical repeat (DHR) protein monomers and protein-Ca2+ supramolecular assemblies directly nucleated nano-calcite particles with non-natural {110} or {202} faces, subsequently assembling into oriented calcite mesocrystals. Nucleation throughout the solution was observed as well as near the sheet-like Ca2+-incubated supramolecular assemblies. It was determined that nanocrystal size and polymorph could be tailored by varying the length and surface chemistry of the protein templates. The structural regularity and tunability of the DHR proteins allowed for comprehensive investigation of the interface driving the templating for nucleation, with implications for hybrid organic-inorganic materials with novel functions such as carbon sequestration.
Reference:
Fatima A. Davila-Hernandez, Biao Jin, Harley Pyles, Shuai Zhang, Zheming Wang, Timothy F. Huddy, Asim K. Bera, Alex Kang, Chun-Long Chen, James J. De Yoreo & David Baker, Nature Communications 14 8191 (2023) DOI: 10.1038/s41467-023-43608-1
Full paper Copyright © 2024 Springer Nature Limited
View All News