How do AuPd nanoparticles respond to high-temperature gas environments?
Alexandre Foucher, Eric Stach, and their colleagues at the University of Pennsylvania and Harvard University published recent work using the Hummingbird Scientific in-situ gas cell TEM sample holder to investigate the effect of high temperature O2, H2, air, CO and CO2 environments on the restructuring of Au0.75Pd0.25 catalyst nanoparticles. The study supported spectroscopic analysis with atomic resolution STEM and density functional theory (DFT) calculations.
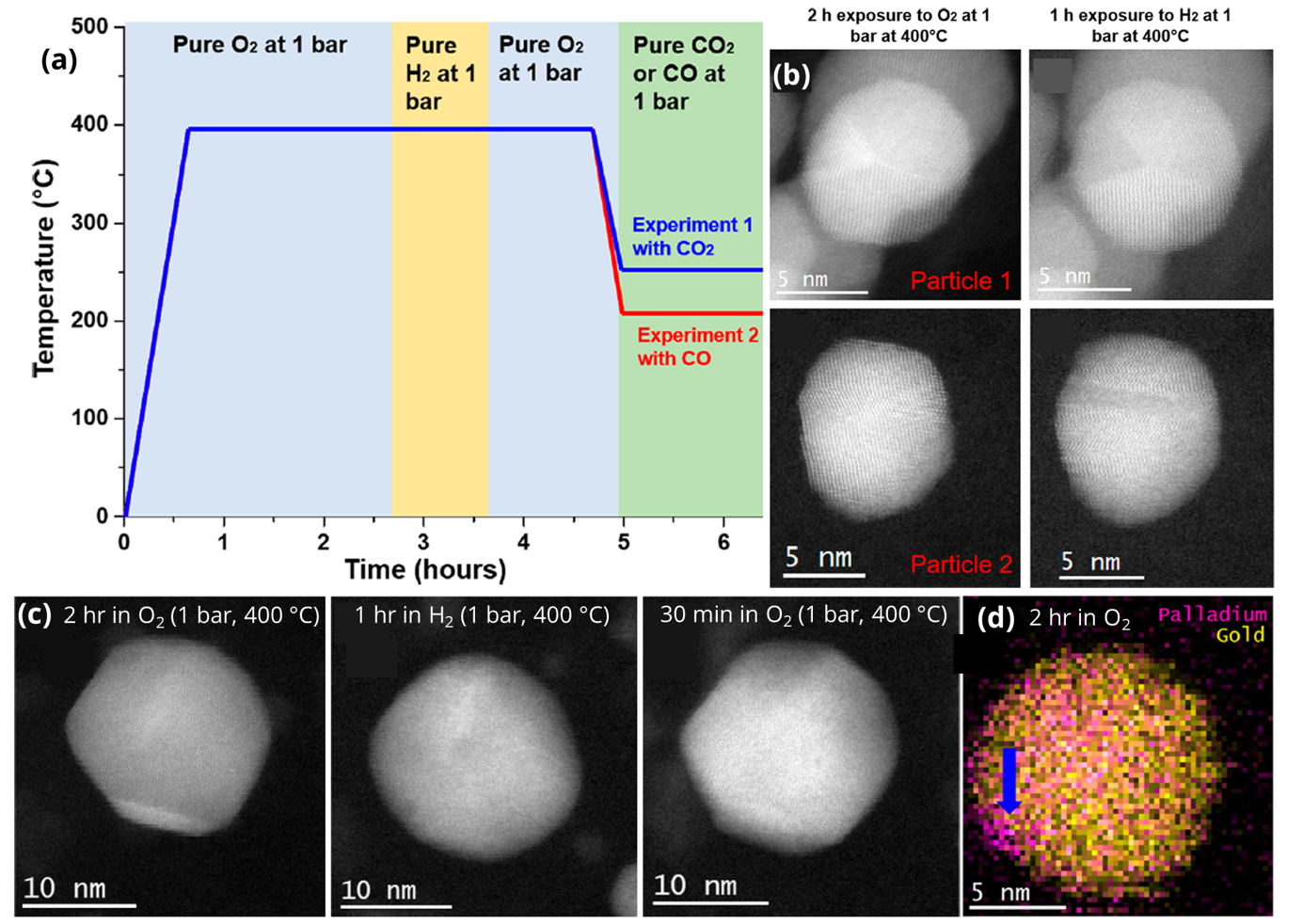
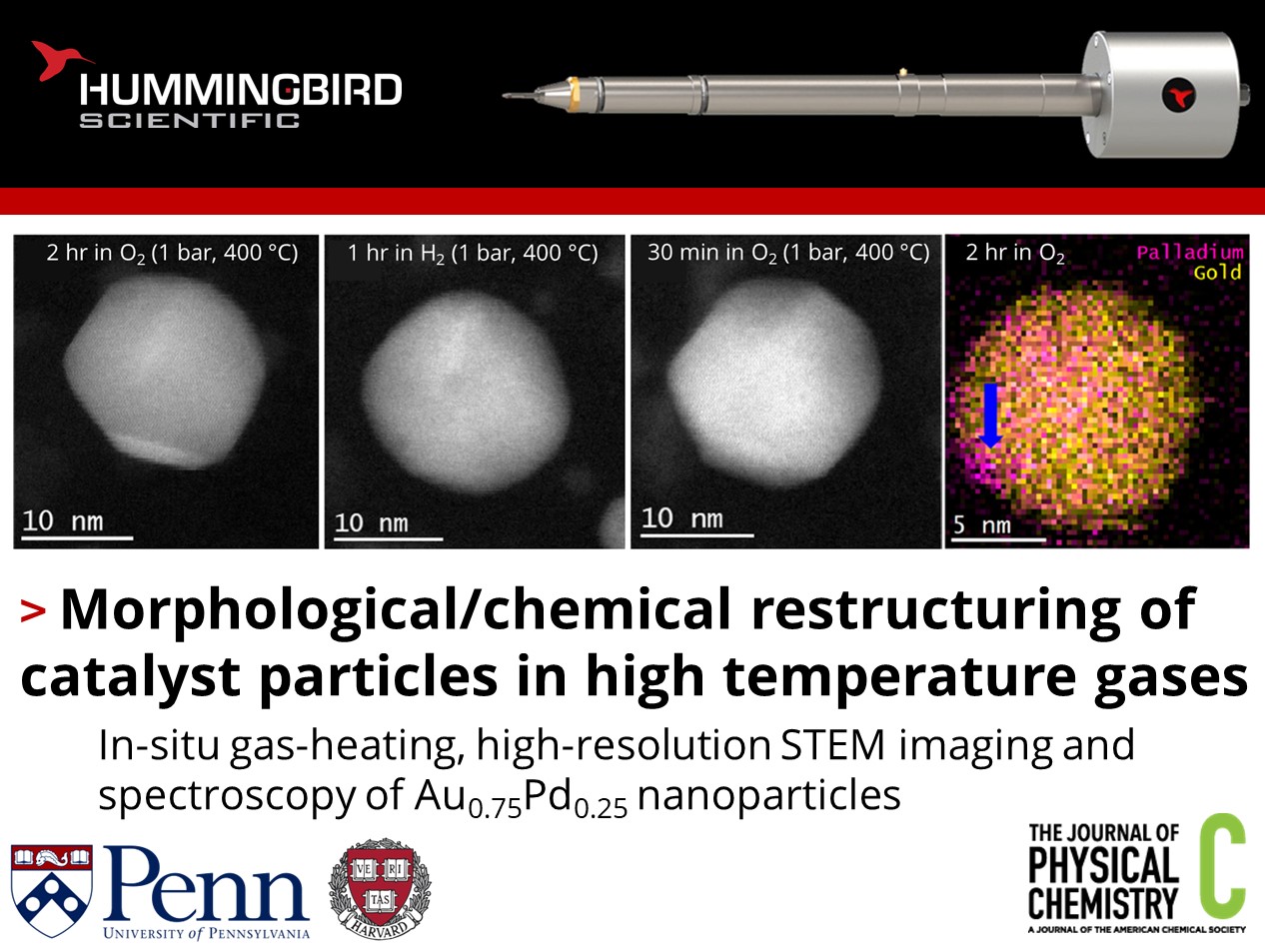
a) Summary of temperature and gases used for series of experiments. b) Atomic resolution STEM comparing post-O2 exposure and post-H2 exposure showing facets vs rougher faces c) Loss and return of surface facets after re-exposure to O2 400 °C. d) EDS map showing segregation of Pd into “islands” after exposure to O2 at 400 °C. Copyright © 2022 American Chemical Society
Using in-situ scanning transmission electron microscopy (STEM) with high-resolution energy dispersive X-ray spectroscopy (EDS) and electron energy loss spectroscopy (EELS) under gaseous environment and heating, the evolution of nanoparticle morphology and chemistry were analyzed. Air and O2 at 400 °C led to sharpening of the facets of particles, with small areas of segregated Pd. At 200 °C, CO exposure led to the loss of facets, while H2 exposure at 400 °C led to subtle increases to surface roughness. This is backed up by DFT calculations, which suggest that the reduction of PdO to Pd causes smaller facets and rougher edges. The study reveals complex interactions between gas molecules, electron beam, and the surface energy of nanoparticles and their facets, contributing to our understanding of catalyst restructuring in different environments and the effect on catalytic activity.
Reference:
Alexandre C. Foucher, Cameron J. Owen, Tanya Shirman, Joanna Aizenberg, Boris Kozinsky, and Eric A. Stach, J. Phys. Chem. C 126 (42) 18047-18056 (2022) DOI: 10.1021/acs.jpcc.2c05929
Full paper Copyright © 2022 American Chemical Society
View All News