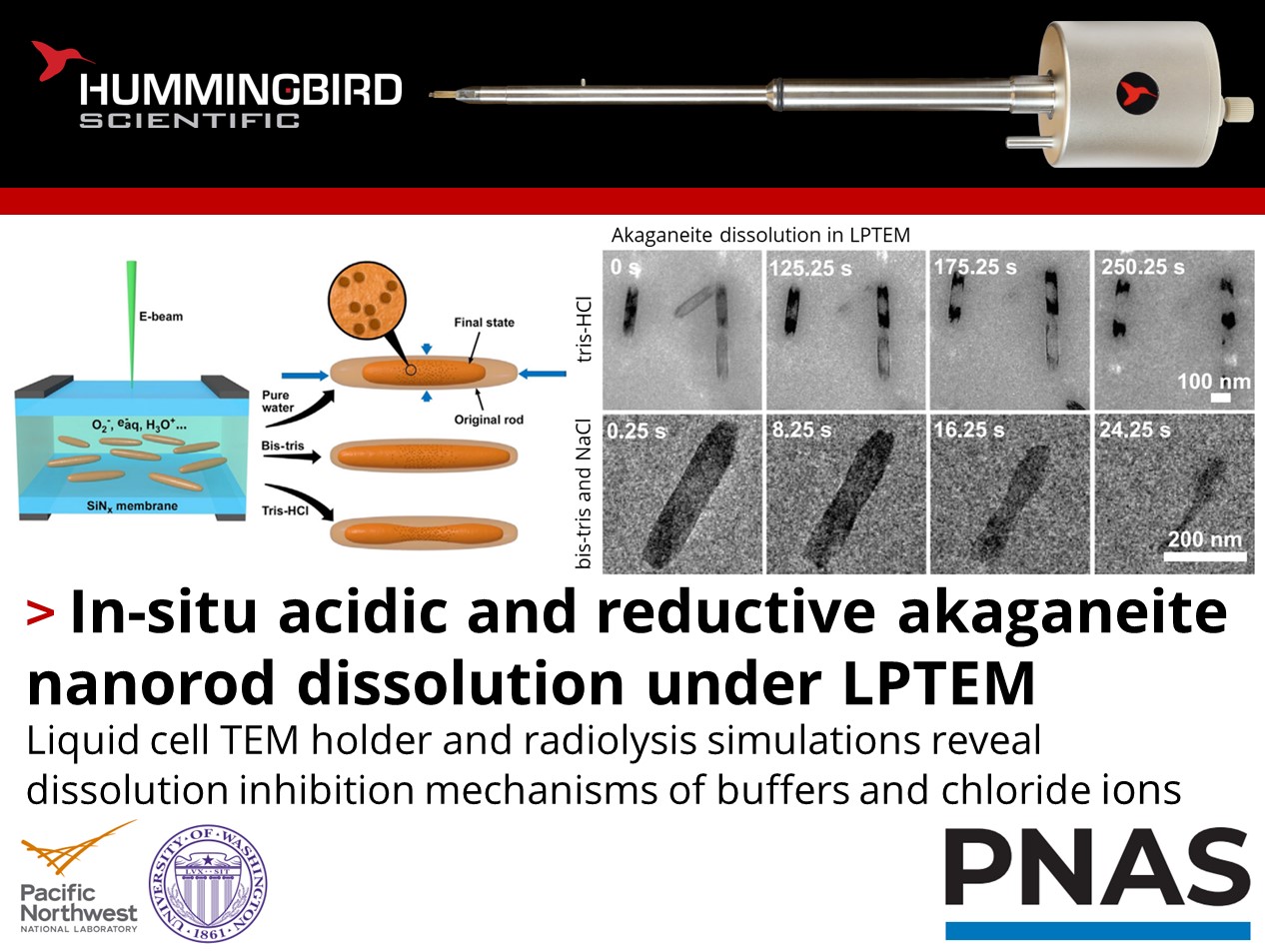
How can both acidic and reductive solution chemistry slow particle dissolution?
Lili Liu, James DeYoreo, and their team at the University of Washington and Pacific Northwest National Laboratory recently published an exciting study using their Hummingbird Scientific in-situ TEM liquid cell sample holder to investigate the dissolution mechanisms of akaganeite (β-FeOOH) nanorods. Liquid-phase transmission electron microscopy (LP-TEM) was combined with radiolysis simulations to image dissolution evolution and measure dissolution rates of the nanorods in different solutions.
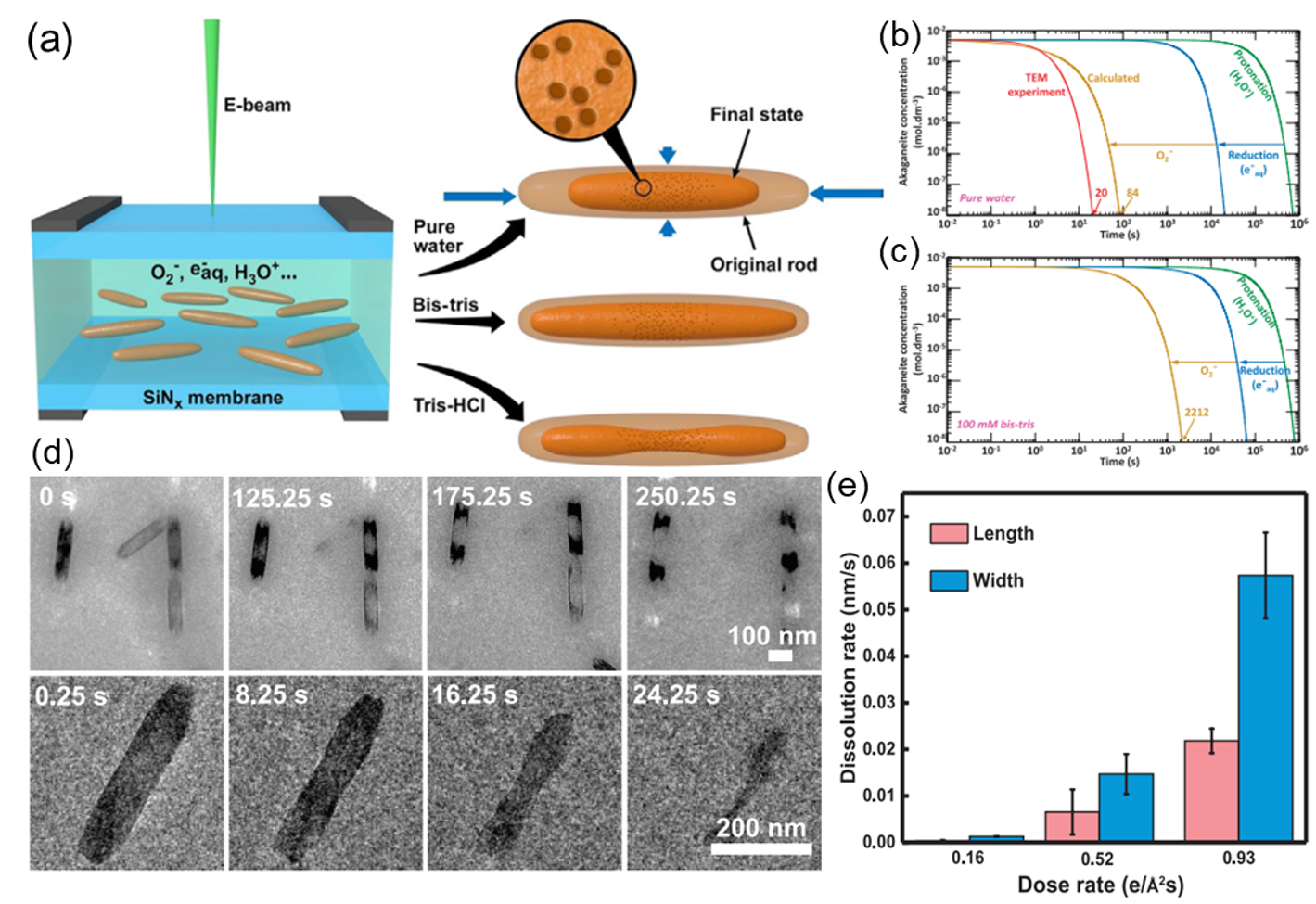
Figures showing a) Conceptual model of dissolution morphologies and evolutions observed. b) Predicted time evolution of the concentration of akaganeite in pure, neutral, pH = 7 water irradiated by an electron beam at a dose rate of 0.53 e−/Å2s. c) Predicted time evolution of the concentration of akaganeite in 100 mM bis-tris irradiated by an electron beam at a dose rate of 0.53 e−/Å2s. d) Time evolution of akaganeite dissolution observed in 100 mM bis-tris and 50 mM NaCl at a dose rate of 1.47 e−/Å2s (upper) and 20 mM tris-HCl at a dose rate of 0.86 e−/Å2s (lower). e) Comparison of measured dissolution rates along the width and length in 20 mM tris-HCl. Copyright © 2023 the Author(s). Published by PNAS. This open access article is distributed under Creative Commons Attribution-NonCommercial–NoDerivatives License 4.0 (CC BY-NC-ND).
With radiolytic simulations as a guide, these dissolution rates were varied systematically using pH buffers, background chloride anions, and electron beam dose. While buffers inhibited dissolution by via consumption of radiolytic species, chloride anions suppressed dissolution at rod tips and promoted dissolution at rod sides via complex surface and structural evolution. The Hummingbird Scientific liquid holder facilitated the observation of anisotropic dissolution along different crystallographic directions, revealing the interplay between acidic and reductive dissolution processes. The results will inform future in-situ studies concerning the nanoscale mechanisms that govern metal cycling in natural environments.
Reference: Lili Liu, Michel Sassi, Xin Zhang, Elias Nakouzi, Libor Kovarik, Sichuang Xue, Biao Jin, Kevin M. Rosso, and James J. De Yoreo. PNAS 120 (23) e2101243120 (2023). DOI: 10.1073/pnas.2101243120
Full paper Copyright © 2023 the Author(s). Published by PNAS. This open access article is distributed under Creative Commons Attribution-NonCommercial-NoDerivatives License 4.0 (CC BY-NC-ND).
View All News