How can the SEM Bulk Liquid Electrochemistry sample holder unlock real-time insights into nanoscale electrochemical transformations?
Phillipp Grosse, See Wee Chee, Beatriz Roldan Cuenya, and colleagues at the Fritz-Haber-Institute of the Max-Planck Society published on their use of the Hummingbird Scientific in-situ SEM bulk liquid electrochemistry sample holder to capture reversible growth and dissolution of Cu nanocubes, directly correlating redox cycling with morphological evolution.
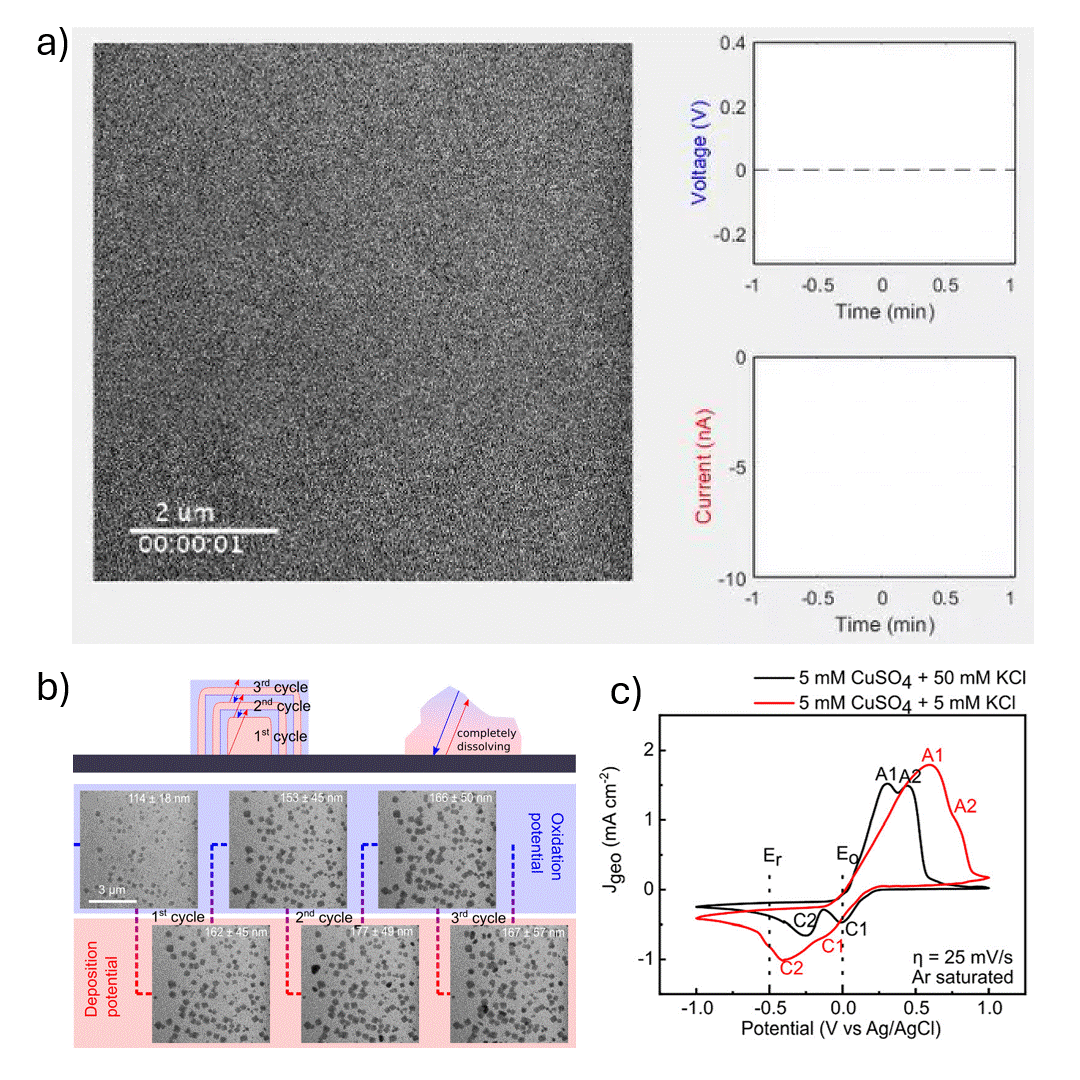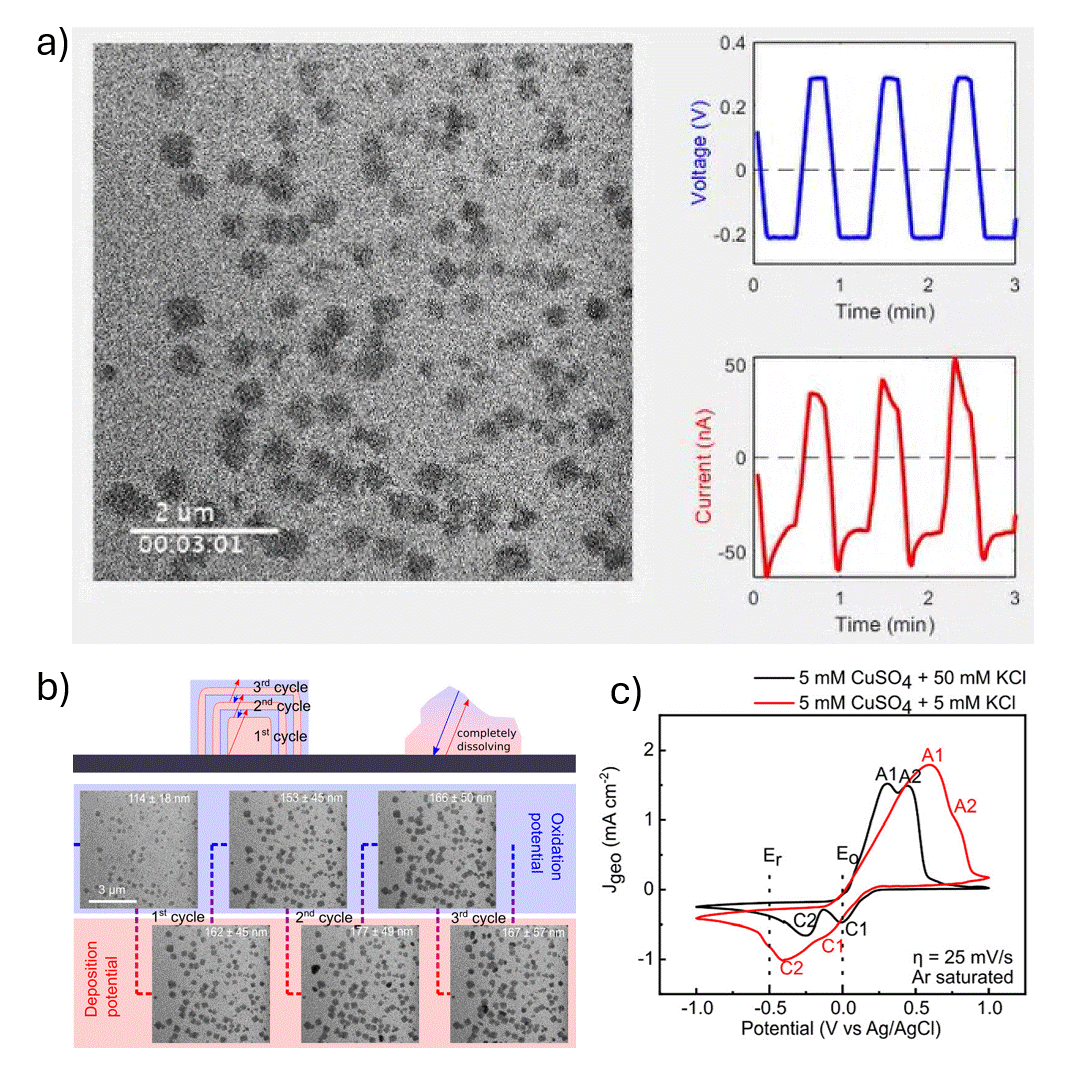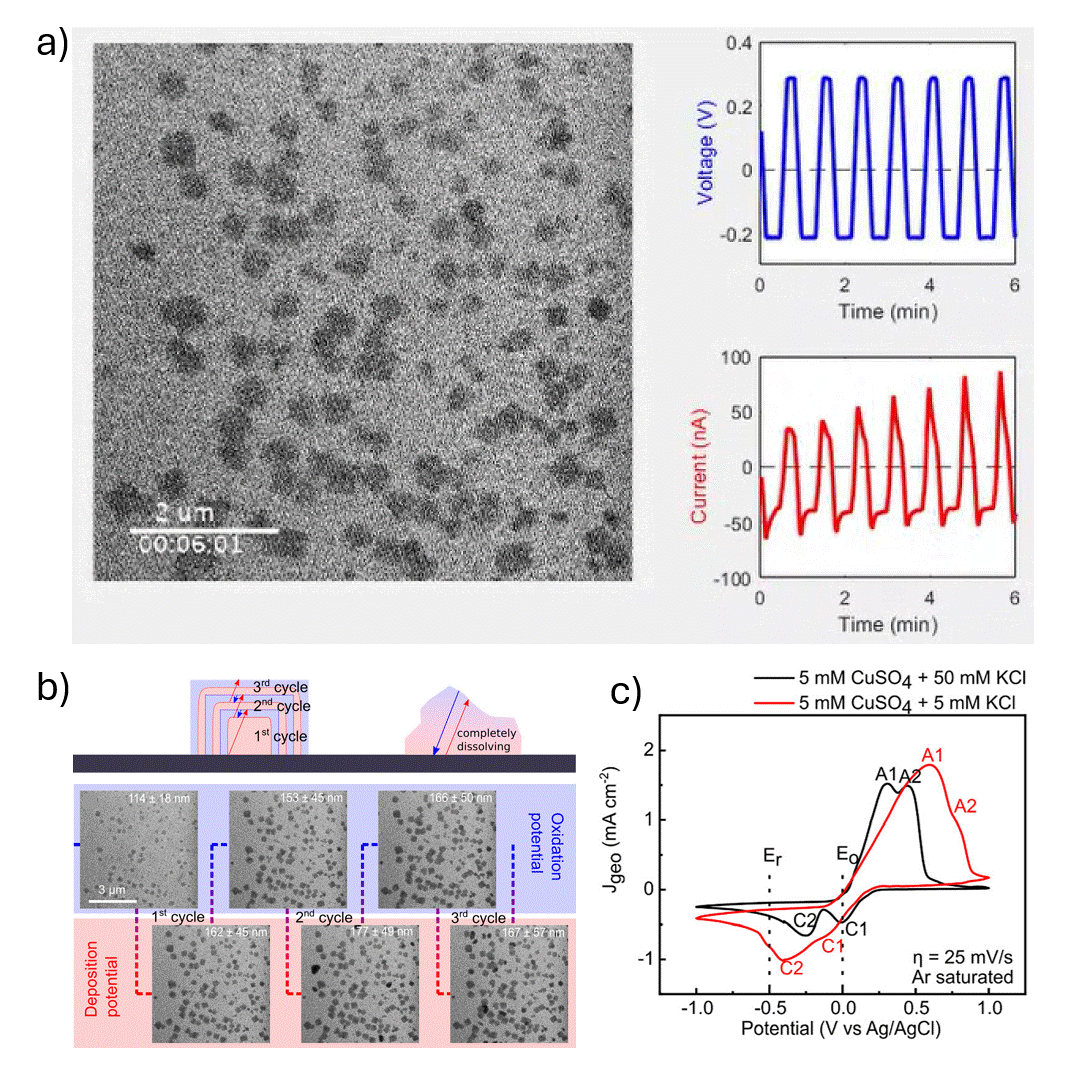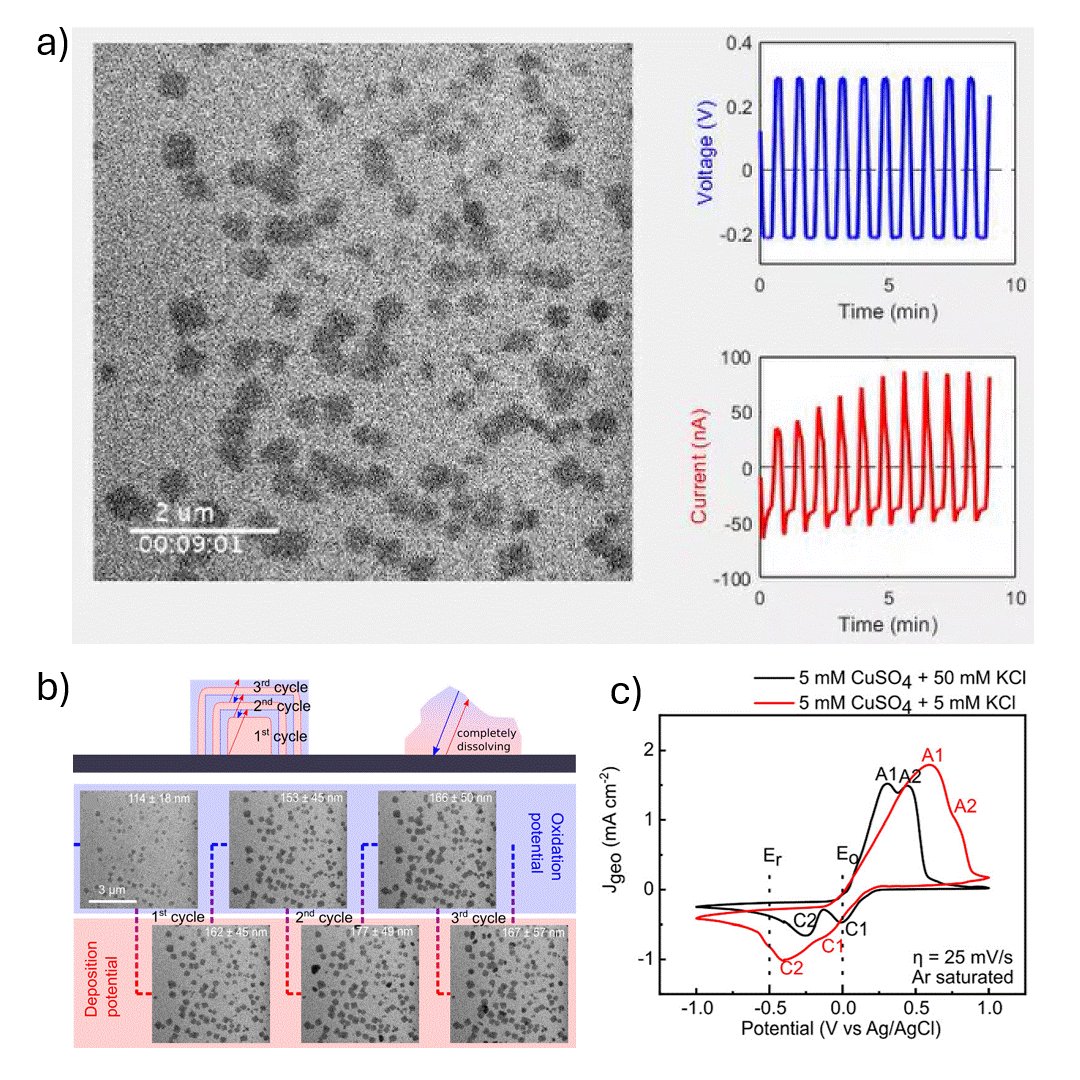
In-situ SEM visualization of Cu nanocube nucleation and growth during electrochemical cycling. a) In-situ video showing controlled formation of Cu cubes during a potential sweep, with size and shape evolving over multiple cycles. b) (top) Proposed mechanism of Cu cube growth via electrodeposition, with layer formation during reduction (Er) and shrinkage during oxidation (Eo); non-cubic particles dissolve during Eo. (bottom) Operando SEM images from cycles 2–4 in 5 mM CuSO₄ + 5 mM KCl during electrochemical synthesis. c) Cyclic voltammograms in 5 mM CuSO₄ with 5 mM or 50 mM KCl at 25 mV s⁻¹, showing current responses at Er and Eo. Anodic peaks A1 and A2 correspond to Cu₂O and CuO formation; cathodic peaks represent their reduction.
The authors investigated the electrodeposition of copper (Cu) nanocubes with well-defined {100} facets on glassy carbon substrates to explore their potential in electrochemical CO₂ reduction (CO₂RR). They systematically optimized key parameters—such as chloride ion concentration, reduction-to-oxidation time ratios, and the number of deposition cycles—to control the morphology, size, and surface coverage of the Cu nanocubes. The synthesis was carefully tuned using cyclic voltammetry, and the resulting morphological evolution was monitored in real time using in situ electrochemical scanning electron microscopy (EC-SEM). Ex situ transmission electron microscopy (TEM) and electron diffraction were used to confirm structural details, revealing that the cube formation followed a nucleation-driven growth process with subsequent layered deposition and partial dissolution in later cycles.
The Hummingbird Scientific in-situ SEM bulk liquid electrochemistry sample holder was a key experimental enabler allowing direct visualization of nanoscale redox dynamics under realistic electrochemical conditions. This setup provided crucial insight into the growth and dissolution behavior of Cu nanostructures during potential cycling. The findings highlight the importance of both electrolyte composition and electrochemical parameters in achieving controlled nanostructure formation, offering valuable guidance for tailoring electrocatalyst morphology to improve product selectivity and efficiency in CO₂RR and other electrochemical applications.
Reference: Philipp Grosse, Aram Yoon, Clara Rettenmaier, See Wee Chee, and Beatriz Roldan Cuenya, The Journal of Physical Chemistry C 124, 49 (2020). DOI: 10.1021/acs.jpcc.0c09105
Full paper From The Journal of Physical Chemistry C under the Creative Commons license
View All News

