How can perovskite oxide surfaces be altered to improve catalytic properties?
SungHyun Jeon, Bong-Joong Kim, and WooChul Jung from KAIST, Gwangju Institute of Science and Technology, Northwestern University, Sungshin Women’s University, Korea Institute of Energy Research, and Seoul National University published recent work characterizing Cu-substituted La0.6Sr0.4Co0.2Fe0.8O3-δ (LCSF) perovskite oxide catalyst evolution during oxygen reduction reaction (ORR) using the Hummingbird Scientific gas heating TEM sample holder. Microstructural and chemical changes of the doped and un-doped surface during oxygen reduction were characterized by in-situ transmission electron microscopy (TEM), scanning transmission electron microscopy with energy dispersive X-ray spectroscopy (STEM-EDS), and ex-situ scanning electron microscopy (SEM).
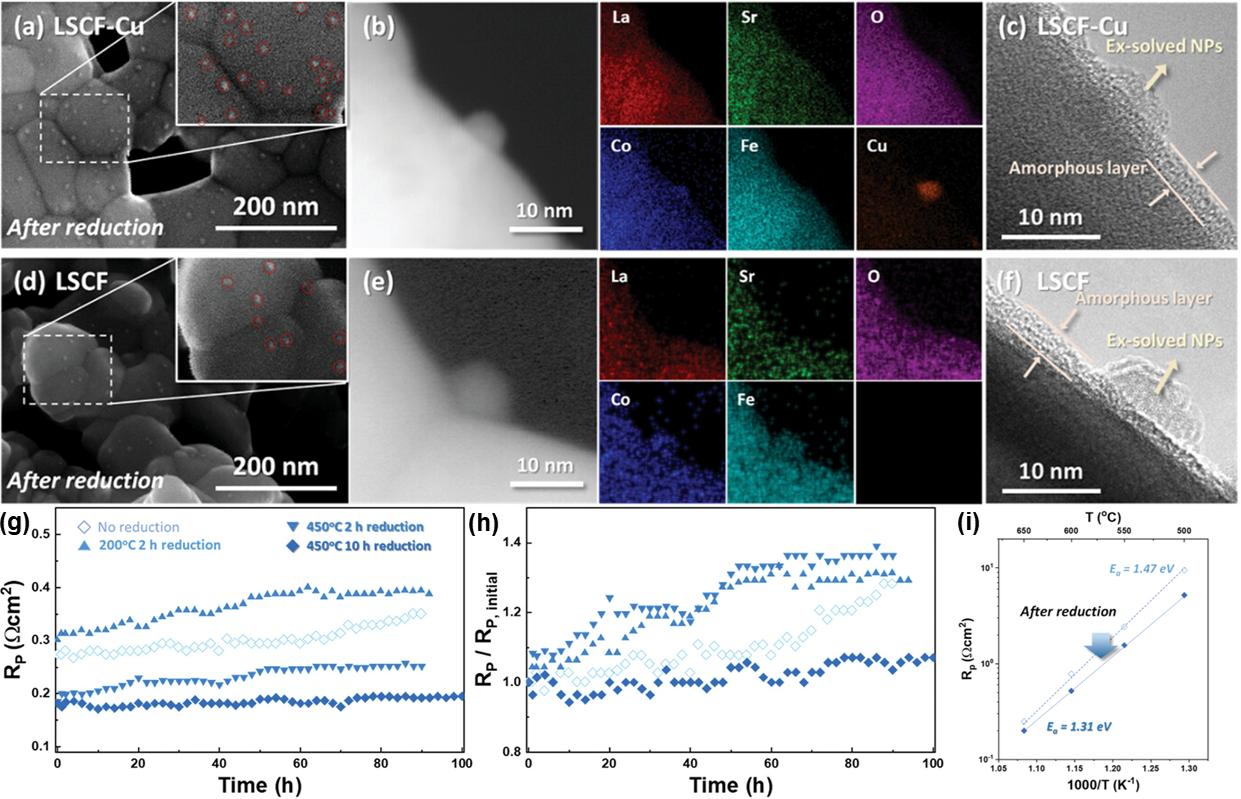
Comparison of ex-solution behavior according to Cu doping: a) SEM, b) HAADF-STEM & EDS, and c) TEM results of doped LSCF-Cu. d) SEM, e) HAADF-STEM & EDS, and f) TEM results of undoped LSCF. g) Long-term stability test at 650 °C. h) Degree of degradation by changing the y-axis of g) to RP/RP, initial. i) Arrhenius plot for LSCF-Cu with and without reduction. Copyright © 2024 The Author(s). Advanced Materials published by Wiley-VCH GmbH
In-situ imaging under TEM at elevated temperatures revealed concurrent surface amorphization of the perovskite alongside the formation of multicomponent metal nanocatalysts which decorate the surface. After an extended period of time, the amorphous layer is resistant to a high-temperature oxidizing atmosphere, leading to significant increase in the electrode resistance to ORR without significant degradation. Compared to undoped perovskite which forms Co-Fe nanocatslysts, Cu-dominated Cu-Co-Fe nanocatalysts form at a higher density, while the Cu-doped material formed amorphous layers of similar thickness. The fabricated metal-based perovskite oxide electrode demonstrated a remarkable improvement in activity and durability, with applications in high-temperature redox-dynamic nanoarchitecture catalyst structures.
Reference:
SungHyun Jeon, Wan-Gil Jung, Hohan Bae, Sejong Ahn, Bonjae Koo, WonJeong Yu, Seunghyun Kim, DongHwan Oh, Uisik Kim, Scott A. Barnett, Jongsu Seo, Bong-Joong Kim, WooChul Jung, Advanced Materials 36 (40) 2404103 (2024) DOI: 10.1002/adma.202404103
Full paper Copyright © 2024 The Author(s). Advanced Materials published by Wiley-VCH GmbH
View All News

