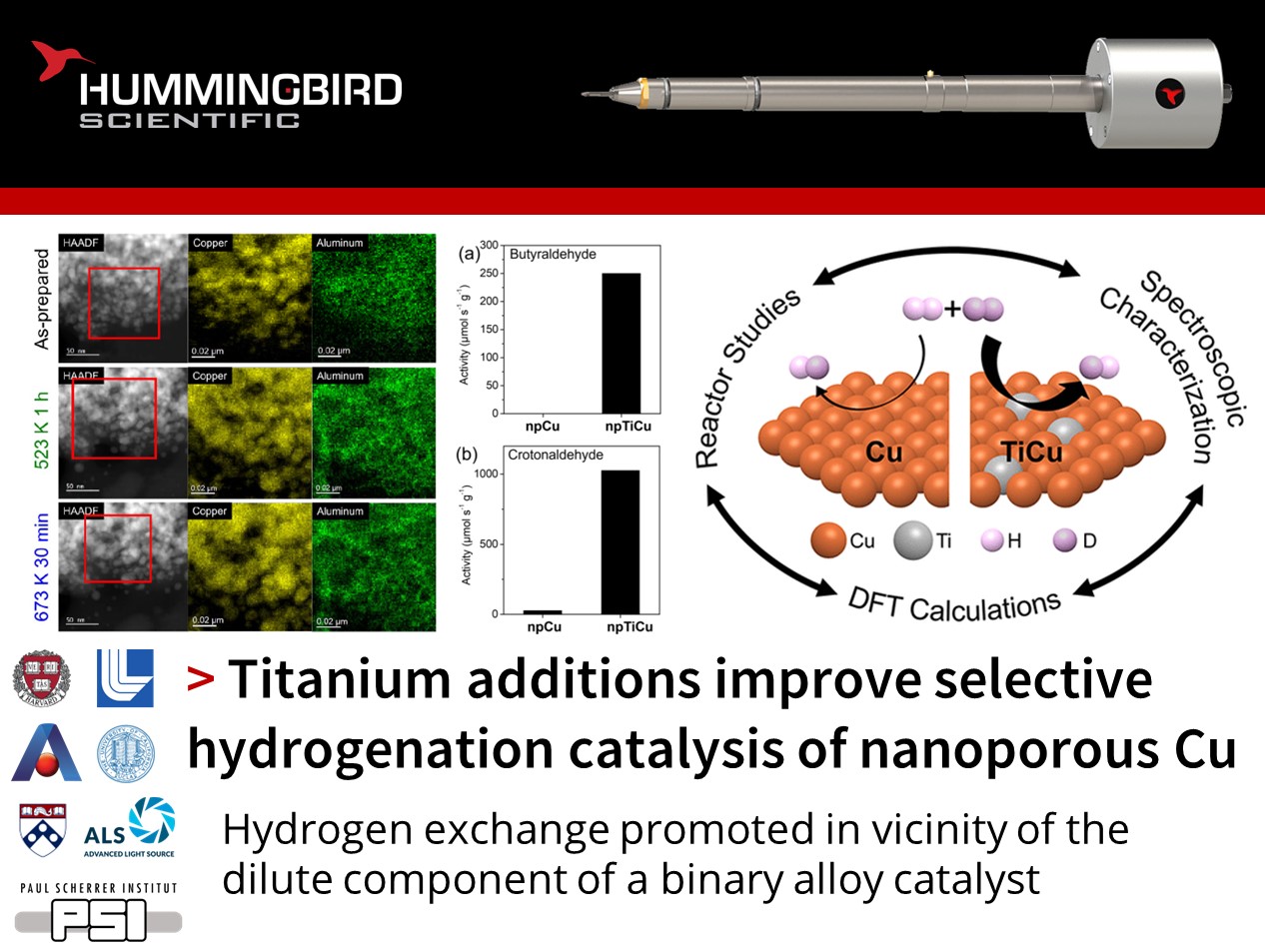
How can alloying be used to improve metal catalysts?
Jennifer Lee, Jurgen Biener, and their colleagues at Harvard University and Lawrence Livermore National Laboratory published an exciting study using their Hummingbird Scientific in-situ TEM gas-environmental heating sample holder to demonstrate the use of titanium alloying to promote hydrogen dissociation over nanoporous copper catalysts.
Combining in situ scanning transmission electron microscopy (STEM), electron energy loss spectroscopy (EELS), energy dispersive X-ray spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), and X-ray absorption spectroscopy (XAS) the reduction of copper and titanium oxides to metallic states under a reducing hydrogen environment at elevated temperatures was confirmed. Complementary density functional theory (DFT) calculations suggest isolated titanium atoms at the catalyst surface facilitate hydrogen dissociation and recombination compared to pure copper. This led to an increase in the measured H2-D2 exchange rate, which was attributed to a change of rate-limiting step from dissociative adsorption on copper to hydrogen recombination on titanium. The reaction rates and selectivity of a typical hydrogenation reaction were greatly improved when Ti-doped catalysts were used.
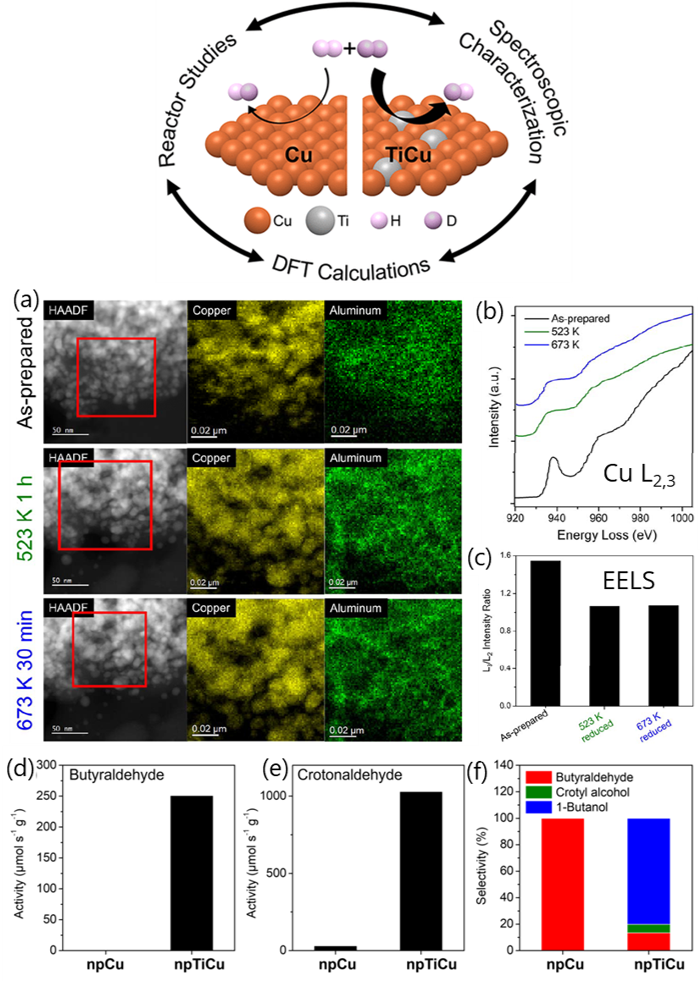
Figures showing a) In-situ HAADF-STEM and EDS elemental mapping of Cu and Al in boxed area for as-prepared npTiCu and after exposed to H2 at 523 K for 1 h (green), and 673 K for 30 min (blue), showing the coarsening of the nanopore ligaments during hydrogenation. b) In-situ EELS spectra of Cu L2,3 edges of as-prepared npTiCu and after exposure to H2 at 523 K for 1 h, and 673 K for 30 min, demonstrating that the Cu has ben reduced by the hydrogen. c) Corresponding L3/L2 intensity ratio. Reaction rates for hydrogenation of d) butyraldehyde and e) crotonaldehyde as well as f) selectivity for crotonaldehyde hydrogenation were greater in NpTiCu than in npCu pretreated at 523 K.
This combined correlative microscopy, spectroscopy and computational study provides valuable insights into tuning hydrogen activation over single-atom alloy catalysts. Critically, the use of in situ STEM-EELS allowed direct observation of the catalyst structural evolution under reducing conditions relevant to catalysis.
Reference: Jennifer D. Lee, Zhen Qi, Alexandre C. Foucher, Hio Tong Ngan, Kevin Dennis, Jun Cui, Ilia I. Sadykov, Ethan J. Crumlin, Philippe Sautet, Eric A. Stach, Cynthia M. Friend, Robert J. Madix, and Juergen Biener
Journal of the American Chemical Society 2022 144 (37), 16778-16791 DOI: 10.1021/jacs.2c00830, Full paper Copyright © 2023 American Chemical Society
View All News