Hummingbird Scientific was the first company to provide a commercially-available holder capable of supporting liquid-electrochemical experiments conducted inside the TEM. In subsequent years, we have continued to refine the holder’s capabilities. In its current configuration, the holder enables electrochemical research via integrated biasing contacts that connect to our specially-patterned electrochemical chips, which are available in a variety of materials. Among other topics, the system has been used for key research into battery materials (see Featured Research below).
The analytical electrochemistry tip features the sample basic capabilities of the standard electrochemistry liquid cell, but it also keeps the electrical contacts outside the experimental space. This option includes an optional low-noise potentiostat and fully shielded cabling for maximum analytical capabilities.
Wiring configurations
- Standard configuration: In the standard configuration, the system’s wires are not individually shielded. This configuration can connect to Hummingbird’s heater control box as well as a variety of electronic source and measurement devices.
- Low current/noise: For very small current or voltage values, we offer an individually-shielded, low-noise wiring system. In this system, a shielded coaxial cable runs through the holder shaft and connects through special micro-coax connectors in the holder. The system can be configured for compatibility with a range of potentiostats and power supplies. It is particularly well-suited for small-scale in-situ liquid electrochemistry measurements.
Biologic SP-200 potentiostat
 We are proud to collaborate with Bio-Logic to offer the Bio-Logic SP-200 potentiostat as a research-grade measurement tool for electrochemistry experiments. Hummingbird Scientific’s liquid-electrochemistry and Biologic’s SP-200 potentiostat can be used in combination for corrosion experiments, electrochemistry, electrolysis, and battery and photovoltaic research.
We are proud to collaborate with Bio-Logic to offer the Bio-Logic SP-200 potentiostat as a research-grade measurement tool for electrochemistry experiments. Hummingbird Scientific’s liquid-electrochemistry and Biologic’s SP-200 potentiostat can be used in combination for corrosion experiments, electrochemistry, electrolysis, and battery and photovoltaic research.
Observation of redox product in lithium-oxygen battery
Lithium-oxygen batteries have exceptional higher energy densities. However, the byproduct lithium peroxide, cannot be easily decomposed during the charging cycle. Now, the work led by researchers from Johns Hopkins University have used Hummingbird Scientific’s liquid electrochemistry TEM holder to demonstrate that the decomposition of lithium peroxide can be improved by adding redox mediators as charge-transfer agents. Their findings are published in the recent issue of Advanced Materials. The fundamental understanding of the lithium-oxygen electrochemistry presented in this work may enable the development of better batteries
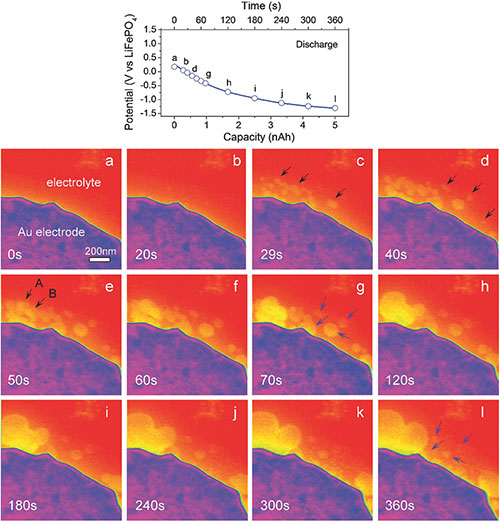
Reference: Mingwei Chen et al. Direct Observations of the Formation and Redox-Mediator-Assisted Decomposition of Li2O2 in a Liquid-Cell Li–O2 Microbattery by Scanning Transmission Electron Microscopy. Advanced Materials (2017). Abstract

