The Generation V liquid-electrochemistry in-situ TEM holder uses a completely newly developed hardware system and optimized electrochemistry chips with configuration of working electrode (WE), counter electrode (CE) and reference electrode (RE) that for the first time replicate bulk electrochemical conditions in-situ in the TEM . This was validated using several model electrochemical systems.
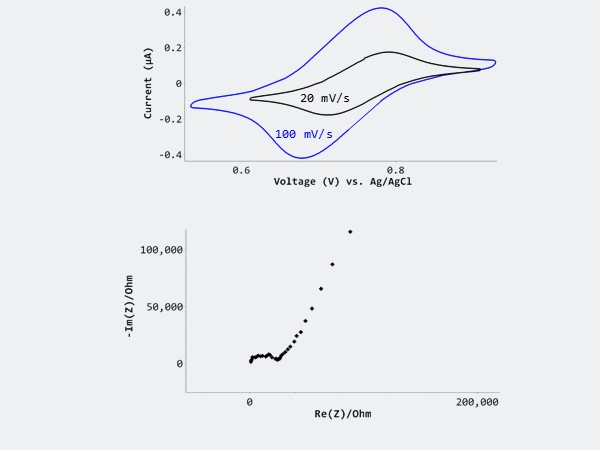
We performed cyclic voltammetry studies in a model 20 mM K3(Fe(CN)6/20 mM K4Fe(CN)6 in 0.1M KCl solutions. The redox reaction of 20 mM ferrocyanide/ 20 mM ferricyanide in 0.1M KCl at different voltage scans show reversible electrode reaction during both the forward and reverse scans, elucidating bulk behavior. The corresponding electrochemical impedance spectroscopy (EIS) measurements shows lower capacitive current, and better signal-to-noise ratio with the lower concentration of solution.
Left image: Top shows CV cycle performed at various potential scan rates – 20 mV/s and 100 mV/s. Bottom shows the corresponding EIS spectrum.
HBS internal data obtained in collaboration with William C. Chueh group at Stanford University.