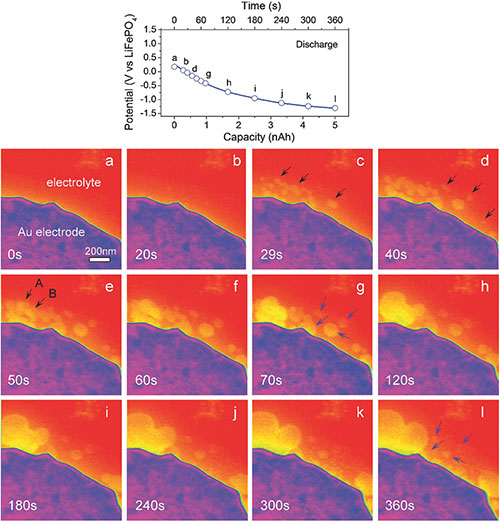
| Nikhilendra Singh, Timothy S. Arthur, Oscar Tutusaus, Jing Li, Kim Kisslinger, Huolin L. Xin, Eric A. Stach, Xudong Fan, and Rana Mohtadi. “Achieving High Cycling Rates via In-situ Generation of Active Nanocomposite Metal Anodes.” ACS Applied Energy Materials (2018) |
Abstract |
| Mei Sun, Xing Li, Zhiqiang Tang, Xianlong Wei and Qing Chen. “Constant-Rate Dissolution of InAs Nanowires in Radiolytic Water Observed by In situ Liquid Cell TEM.” Nanoscale (2018) |
Abstract |
| Chen Houa, Jiuhui Hanb, Pan Liua, Chuchu Yangb, Gang Huangb, Takeshi Fujitab, Akihiko Hiratab, and Mingwei Chen. “Operando observations of RuO2 catalyzed Li2O2 formation and decomposition in a Li-O2 micro-battery,” Nano Energy (2018) |
Abstract |
| Pan Liu, Jiuhui Han, Xianwei Guo, Yoshikazu Ito, Chuchu Yang, Shoucong Ning, Takeshi Fujita, Akihiko Hirata and Mingwei Chen. “Operando characterization of cathodic reactions in a liquid-state lithium-oxygen micro-battery by scanning transmission electron microscopy,” Scientific Reports (2018) |
Abstract |
| Edward R. White, Jared J. Lodico & B. C. Regan. “Intercalation events visualized in single microcrystals of graphite,” Nature Communications (2017) |
Abstract |
| Chuchu Yang, Jiuhui Han, Pan Liu, Chen Hou, Gang Huang, Takeshi Fujita, Akihiko Hirata, and Mingwei Chen. “Direct Observations of the Formation and Redox-Mediator-Assisted Decomposition of Li2O2 in a Liquid-Cell Li–O2 Microbattery by Scanning Transmission Electron Microscopy,” Advanced Materials (2017) |
Abstract |
| Timothy S. Arthur, Per-Anders Glans, Nikhilendra Singh, Oscar Tutusaus, Kaiqi Nie, Yi-Sheng Liu, Fuminori Mizuno, Jinghua Guo, Daan Hein Alsem, Norman J. Salmon, and Rana Mohtadi. “Interfacial insight from operando sXAS/TEM for magnesium metal deposition with borohydride electrolytes,” Chemistry of Materials (2017) |
Abstract |
| J. R. Vance and S. J. Dillon. “Thermally driven bubble evolution at a heater wire in water characterized by high-speed transmission electron microscopy,” Chemical Communications (2017) |
Abstract |
| J. P. Patterson, L. R. Parent, J. Cantlona, H. Eickhoffa, G. Bareda, J. E. Evansa and N.C. Gianneschia. “Picoliter Drop-On-Demand Dispensing for Multiplex Liquid Cell Transmission Electron Microscopy,” Microscopy & Microanalysis (2016) |
Abstract |
| Omid Sadeghi, Clément Falaise, Pedro I. Molina, Ryan Hufschmid, Charles F. Campana, Bruce C. Noll, Nigel D. Browning, and May Nyman. “Chemical Stabilization and Electrochemical Destabilization of the Iron Keggin Ion in Water,” Inorganic Chemistry (2016) |
Abstract |
| R. R. Unocic, L. Baggetto, G. M. Veith, J. A. Aguiar, K. A. Unocic, R. L. Sacci, N. J. Dudneyb and K. L. Morea. “Probing battery chemistry with liquid cell electron energy loss spectroscopy,” Chem. Communication (2015) |
Abstract |
| R.R. Unocic. “In-situ Liquid S/TEM: Practical Aspects, Challenges, and Opportunities,” Microscopy and Microanalysis (2015) |
Abstract |
| W. Zhang, D.H. Alsem, F. Wang, N. Salmon. “In-Situ Liquid Cell TEM Studies of Electrochemical Reaction in Lithium-Ion Batteries,” Microscopy & Microanalysis (2015) |
Abstract |
| C. Wang. “In situ transmission electron microscopy and spectroscopy studies of rechargeable batteries under dynamic operating conditions: A retrospective and perspective view.” Journal of Materials Research (2015) |
Abstract |
P. Abellan, B. L. Mehdi, L.R. Parent, M. Gu, C. Park, W. Xu, Y. Zhang, I. Arslan, J.G. Zhang, C.M. Wang, J.E. Evans, and N.D. Browning ”
Probing the Degradation Mechanisms in Electrolyte Solutions for Li-Ion Batteries by in Situ Transmission Electron Microscopy” Nano Letter (2014) |
Abstract |
| S.W. Chee, D.J. Duquette, F.M. Ross, and R. Hull. “Metastable Structures in Al Thin Films Before the Onset of Corrosion Pitting as Observed using Liquid Cell Transmission Electron Microscopy,” Micoscopy & Microanalysis (2014) |
Abstract |
| R.R. Unocic, X.G. Sun, R.L. Sacci, L.A. Adamczyk, D.H. Alsem, S. Dai, N.J. Dudney, and K.L. More. “Direct Visualization of Solid Electrolyte Interphase Formation in Lithium-Ion Batteries with In Situ Electrochemical Transmission Electron Microscopy,” Microscopy & Microanalysis (2014) |
Abstract |
| R.L. Sacci, N. Dudney, K. More, L.R. Parent, I. Arslan, N.D. Browning, and R.R. Unocic. “Direct Visualization of Initial SEI Morphology and Growth Kinetics During Lithium Deposition by In-Situ Electrochemical Transmission Electron Microscopy,” Chem. Communication (2014) |
Abstract |
| J.M. Miller, D.H. Alsem, N. Salmon, N.E. Johnson and J.E. Hutchison. “Functionalized Surfaces to Improve Imaging Conditions in Liquid Cell Transmission Electron Microscopy. ” Microscopy & Microanalysi (2014) |
Abstract |
| M. Gu, L.R. Parent, B.L. Mehdi, R.R. Unocic, M.T. McDowell, R.L. Sacci, W. Xu, J.G. Connell, P. Xu, P. Abellan, X. Chen,Y. Zhang, D.E. Perea, J.E. Evans, L.J. Lauhon, J.G. Zhang, J. Liu, N.D. Browning, Y. Cui, I. Arslan, and C.M. Wang. “Demonstration of an Electrochemical Liquid Cell for Operando Transmission Electron Microscopy Observation of the Lithiation/Delithiation Behavior of Si Nanowire Battery Anodes.” Nano Letter (2013) |
Abstract |
| Y.Z. Liu, X.M. Lin, Y.G. Sun, T. Rajh. “In-Situ Visualization of Self-Assembly of Charged Gold Nanoparticles.” J. Am. Chem. Soc. 135:10 (2013) pp. 3764–3767 |
Abstract |
| G. Zhu, Y. Jiang, W. Huang, H. Zhang, F. Lin, and C. Jin. “Atomic Resolution Liquid-Cell Transmission Electron Microscopy Investigations of the Dynamics of Nanoparticles in Ultrathin Liquids,” Chem. Commun. 49 (2013) pp. 10944‒10946 |
Abstract |
| M.H. Nielsen, J.R.I. Lee, Q. Hu, T. Y.-J. Han, and J.J. De Yoreo, “Structural evolution, formation pathways and energetic controls during template-directed nucleation of CaCO3,” Faraday Discuss. 159 (2012) pp. 105–121 |
Abstract |
| D. Li, M.H. Nielsen, J.R.I. Lee, C. Frandsen, J.F. Banfield, and J.J. De Yoreo. “Direction-Specific Interactions Control Crystal Growth by Oriented Attachment,” Science 336:6084 (2012) pp. 1014–1018 |
Abstract |
| E.R. White, M. Mecklenburg, B. Shevitski, S.B. Singer, and B.C. Regan, “Charged nanoparticle dynamics in water induced by scanning transmission electron microscopy,” Langmuir 28:8 (2012) pp. 3695–3698 |
Abstract |
| E.R. White, S.B. Singer, V. Augustyn, W.A. Hubbard, M. Mecklenburg, B. Dunn, and B.C. Regan,“In-Situ Transmission Electron Microscopy of Lead Dendrites and Lead Ions in Aqueous Solution” ACS Nano 6:7 (2012) pp. 6038–6317 |
Abstract |
| K.L. Jungjohann, J.E. Evans, J.A Aguiar , I. Arslan, N.D. Browning. “Atomic-scale imaging and spectroscopy for in-situ liquid scanning transmission electron microscopy.” Microscopy & Microanalysis 18:03 (2012) pp. 621–627 |
Abstract |
| L.R. Parent, D.B. Robinson, T.J. Woehl, W.D. Ristenpart, J.E. Evans, N.D. Browning, I. Arslan “Direct in-situ observation of nanoparticle synthesis in a liquid crystal surfactant template,” ACS Nano 6:4 (2012) pp. 3589–3596 |
Abstract |
| T.J. Woehl , J. E. Evans, I. Arslan, W.D. Ristenpart , N.D. Browning “Direct in-situ determination of the mechanisms controlling nanoparticle nucleation and growth,” ACS Nano 6:10 (2012) pp. 8599–8610 |
Abstract |
| J.E. Evans, K.L. Jungjohann, P.C.K. Wong, P.L. Chiua, G.H. Dutrowa, I. Arslan, N.D. Browning. “Visualizing macromolecular complexes with in-situ liquid scanning transmission electron microscopy” Micron 43:11 (2012) pp. 1085–1090 |
Abstract |
| R.R. Unocic, L. Baggetto, K.A. Unocic, G.M. Veith, N.J. Dudney, and K.L. More. “Coupling EELS/EFTEM Imaging with Environmental Fluid Cell Microscopy.” Microscopy and Microanalysis 18 (Suppl 2), (2012) 1104-1105 |
Abstract |
| K.L. Jungjohann, J.E. Evans, J.A Aguiar , I. Arslan, N.D. Browning. “Atomic-scale imaging and spectroscopy for in-situ liquid scanning transmission electron microscopy.” Microscopy & Microanalysis 18:03 (2012) pp. 621–627. |
Abstract |
| K.L. Jungjohann, J.E. Evans, I. Arslan, N.D. Browning. “Electron Energy Loss Spectroscopy for Aqueous in-Situ Scanning Transmission Electron Microscopy.” Microscopy & Microanaysis 17:S2 (2011) pp. 778–779. |
Abstract |
| J.E. Evans, K.L. Jungjohann, N.D. Browning and I. Arslan. “Controlled Growth of Nanoparticles from Solution with In-Situ Liquid Transmission Electron Microscopy,” Nano Lett. 11:7 (2011) pp. 2809–2813 |
Abstract |
| N. de Jonge, D.B. Peckys, G.J. Kremers, D.W. Piston. “Electron microscopy of whole cells in liquid with nanometer resolution,” PNAS 106:7 (2009) pp. 2159–2164 |
Abstract |
| J.E. Evans, N.D. Browning, “Enabling Direct Nanoscale Observations of Biological Reactions with Dynamic TEM,” Microscopy 62:1 (2013) pp. 147–156 |
Abstract |
| E.R. White, M. Mecklenburg, B. Shevitski, S.B. Singer, and B.C. Regan, “Charged nanoparticle dynamics in water induced by scanning transmission electron microscopy,” Langmuir 28:8 (2012) pp. 3695–3698 |
Abstract |
| R.R. Unocic, L.A. Adamczyk, N.J. Dudney, D.H. Alsem, N.J. Salmon, and K.L. More. “In-Situ Electron Microscopy of Electrical Energy Storage Materials,” ECS Fall Meeting 2010 |
Abstract |
| C.M. Wang, W. Xu, J. Liu, D.W. Choi, B. Arey, L.V. Saraf, J.G. Zhang, Z.G. Yang, S. Thevuthasan, D.R. Baer, and N. Salmon. “In-situ transmission electron microscopy and spectroscopy studies of interfaces in Li ion batteries: Challenges and opportunities,” J. Mater. Res. 25:8 (2010) pp. 1541–1547 |
Abstract |

 We are proud to collaborate with BioLogic to offer the BioLogic SP-200 potentiostat as a research-grade measurement tool for electrochemistry experiments. Hummingbird Scientific’s liquid-electrochemistry and BioLogic’s SP-200 potentiostat can be used in combination for corrosion experiments, electrochemistry, electrolysis, battery and photovoltaic research.
We are proud to collaborate with BioLogic to offer the BioLogic SP-200 potentiostat as a research-grade measurement tool for electrochemistry experiments. Hummingbird Scientific’s liquid-electrochemistry and BioLogic’s SP-200 potentiostat can be used in combination for corrosion experiments, electrochemistry, electrolysis, battery and photovoltaic research.