Researchers from IBM, Princeton University, UCLA and UPenn have demonstrated that adding additives can prevent the growth resulting from unwanted side reactions during the electrochemical processes. The results were acquired using Hummingbird’s Liquid Electrochemistry TEM holder and published in Nano letters.
Copyright © 2018 American Chemical Society
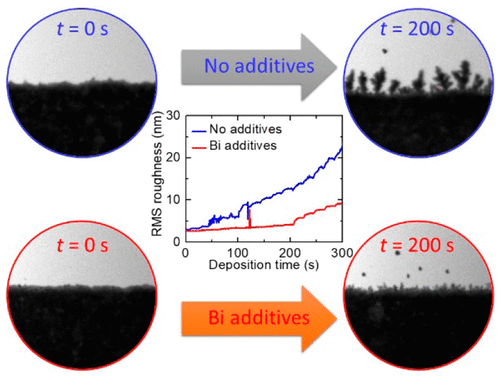
Many electrochemistry systems such as batteries have suffered from unwanted side-reactions, i.e. metal dendritic growth during the electrochemical processes. In some cases, the dendrites formation are directly linked to fire and other safety hazards in the batteries, etc. Here, the researchers were able to inhibit the growth of the dendrites by utilizing inorganic Bi additive during the Zn deposition in ZnAu system. The performance was evaluated using the Hummingbird liquid cell TEM system, which allowed to simultaneous track and quantify the growth front evolution. The results demonstrated that Bi both smooths the surface and inhibits the continued unwanted deposition of Zn in the alloy. This observation using the liquid cell system can be extended to study similar processes in other electrochemical systems
Reference: Jeung Hun Park, Nicholas M. Schneider, Daniel A. Steingart, Hariklia Deligianni, Suneel Kodambaka, and Frances M. Ross. “Control of Growth Front Evolution by Bi Additives during ZnAu Electrodeposition,” Nano Letters (2018). DOI: 10.1021/acs.nanolett.7b04640
View All News

